Session Information
Session Type: Abstract Session
Session Time: 2:00PM-2:15PM
Background/Purpose: T cell mediated pathology is central to many forms of autoimmunity, and cytokines perform critical inflammatory functions during this process. IL-2 and IL-15 are well studied common gamma chain cytokine family members that sharply augment T and NK cell function. Antagonizing both pathways with a single molecule is an attractive therapeutic strategy, especially if the IL-2 function in suppressive Tregs can be spared. Using our computational design platform coupled with directed evolution, we created a de novo IL-2/IL-15 inhibitor that has high affinity for IL-2 receptor beta (IL2RB) with no detectable IL-2 receptor gamma (IL2RG) binding, resulting in an inhibitor that antagonizes both the IL-2 and IL-15 pathways. Further computational engineering strategies were performed to improve the inhibitor’s thermodynamic stability in low pH environments and increase its resistance to proteases. The resulting therapeutic candidate has potential clinical applications that are differentiated from other related biological therapies, enabling local routes of administration and sparing Treg function.
Methods: Computationally derived binding profiles against IL2RB and IL2RG were determined via biolayer interferometry. Protein stability was addressed in the pH range 2.0-8.0 by thermal and/or chaotropic denaturation assays using spectroscopic techniques. Proteolytic resistance was separately assessed by mass spectrometry. Therapeutic candidates with high affinity for IL2RB and hyperstable biophysical properties were assessed for functional antagonism of IL-2 and IL-15 signaling via pSTAT5 flow cytometric analysis of human T and NK cell subsets. In vivo,biophysical properties were assessed by inhibitor quantification in the GI tract of orally dosed mice, and biological anti-inflammatory activity was assessed using a murine delayed-type hypersensitivity (DTH) model.
Results: We report development of a de novo IL-2/IL-15 inhibitor with higher affinity for IL2RB compared to wild type IL-2 (KD < 1nM) and undetectable IL2RG binding via Octet biolayer interferometry. This molecule inhibits IL-2 pSTAT5 signaling in human pan T cells with single digit nM IC50 potency, while Treg cells are still able to utilize IL-2 through pSTAT5 signaling at those concentrations (Figure 1). In vitro, this therapeutic candidate shows better thermodynamic properties at pH 2 and > 10-fold increase in protease resistance compared to a parental agonist molecule. Systemically administered, the IL-2/IL-15 dual inhibitor blocked DTH swelling in mice with statistical significance; orally administered, it retained receptor binding activity in the intestines, demonstrating robust resistance to denaturation and degradation.
Conclusion: Computational design enabled development of a potent, hyperstable inhibitor of both the IL-2 and IL-15 pathways. These biophysical properties may enable novel therapeutic strategies for inflammatory and autoimmune diseases. Furthermore, these results demonstrate that de novo protein design can be readily applied to adjust receptor specificity and affinity to achieve the binding and functional therapeutic profile of interest.
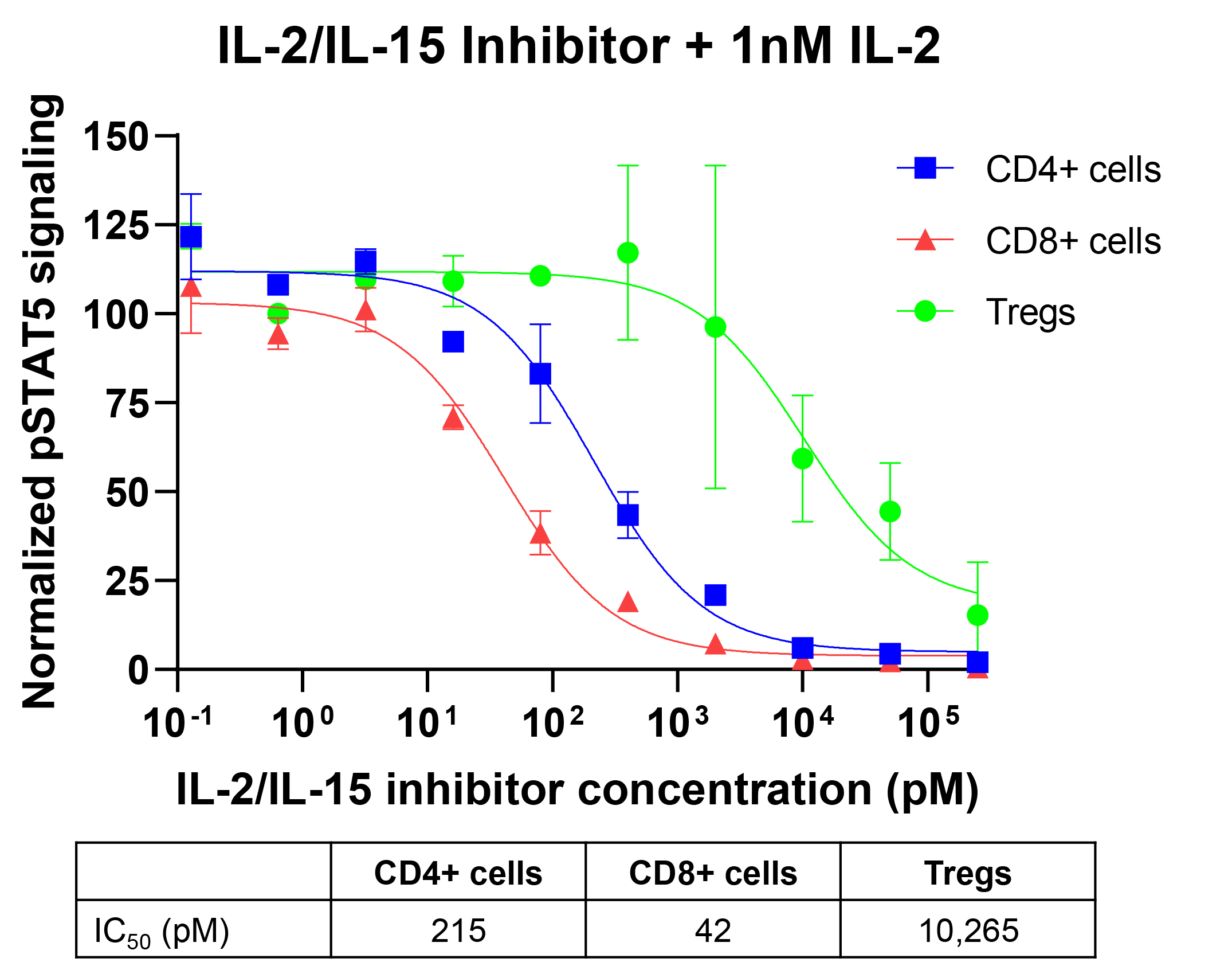 Figure 1. An IL_2/IL_15 dual inhibitor blocks human CD4+ and CD8+ T cell pSTAT5 signaling at concentrations where Tregs demonstrate 100% pSTAT5 signaling (best-fit values).
Figure 1. An IL_2/IL_15 dual inhibitor blocks human CD4+ and CD8+ T cell pSTAT5 signaling at concentrations where Tregs demonstrate 100% pSTAT5 signaling (best-fit values).
To cite this abstract in AMA style:
Vergara R, Chen A, Chen J, Riley M, Blancas-Mejia L, Mortales C, Berrocal T, Priya T, Mason M, Yu K, Sharapova O, Nelson J, Quijano-Rubio A, Linsky T, Swanson R, Silva D. Development of a Computationally Designed, Hyperstable Dual Inhibitor of the IL-2 and IL-15 Receptors: A Novel Therapeutic Candidate for Inflammatory Conditions [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/development-of-a-computationally-designed-hyperstable-dual-inhibitor-of-the-il-2-and-il-15-receptors-a-novel-therapeutic-candidate-for-inflammatory-conditions/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-a-computationally-designed-hyperstable-dual-inhibitor-of-the-il-2-and-il-15-receptors-a-novel-therapeutic-candidate-for-inflammatory-conditions/
