Session Information
Date: Friday, November 6, 2020
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The COVID-19 pandemic has increasingly driven clinical care into remote settings, where patients with immune-mediated and inflammatory diseases struggle to convey symptom severity to their health providers. Smartphone sensor technology has demonstrated the ability to quantitatively measure disease symptoms, however, similar tools for psoriatic arthritis (PsA) are lacking. Here, we describe preliminary data on the performance of 3 novel tools that can be self-administered by patients using the Psorcast iOS app to measure PsA symptoms.
Methods: Three novel smartphone sensor-based measurement tools were developed as part of Psorcast to assess PsA symptoms affecting joints domains (Fig 1). Patients with PsA and controls are being recruited at PPACMAN clinical sites to perform the digital measurements while being concurrently assessed by a rheumatologist.
The Digital Jar Open tool uses the gyroscope to measure inward and outward rotation of each arm to generate an inward symmetry score and outward symmetry score that are normalized within each participant. The 30-Second Walk tool measures gait with the smartphone in a pocket during a walk to generate a symmetry score using PDKit. The Finger/Toe Photo captures finger and toe images, normalizing them with the contralateral nail bed width to measure relative digit widths.
To assess preliminary validity, Digital Jar Open composite symmetry scores were compared between participants with asymmetric upper extremity tenderness as determined by the examining physician and those with none. Triaxial gait asymmetry from the 30-Second Walk was compared between participants with diagnosed asymmetric lower extremity joint tenderness and those with none. The relative widths of digits captured by the Finger/Toe Photo were compared to matching digits from the rest of the cohort with this data, and the resulting data was grouped by physician diagnosis of dactylitis.
Results: In all, 26 patients have been recruited to date. Of these, 15, 15, and 7 provided data to assess the Digital Jar Open, 30-Second Walk, and Finger/Toe Photo tools, respectively. Results from both the Digital Jar Open test and 30-Second Walk demonstrated that the asymmetric joint pain groups are markedly different from controls, but neither of these findings reach statistical significance (Fig. 2A). Finally, 3 individuals in our cohort had dactylitis, all in toes 2 and 3. The relative widths of these digits compared to the rest of the cohort showed a statistically significant separation between the two groups near the 10% difference mark (Fig. 2C). Interestingly, this is the validated cutoff for diagnosing dactylitis from the Leeds Dactylometer.
Conclusion: While validation of Psorcast measurements will require an expanded cohort and analysis beyond symmetry-related features, we observed that the 3 sensor-based measurements described here can distinguish some clinical features of PsA. If validated, these and other Psorcast tools may provide for remote self-assessment when clinical visits cannot be performed. Importantly, longitudinal and frequent symptom measurement could be of high value to study disease progression and assessing treatment response.
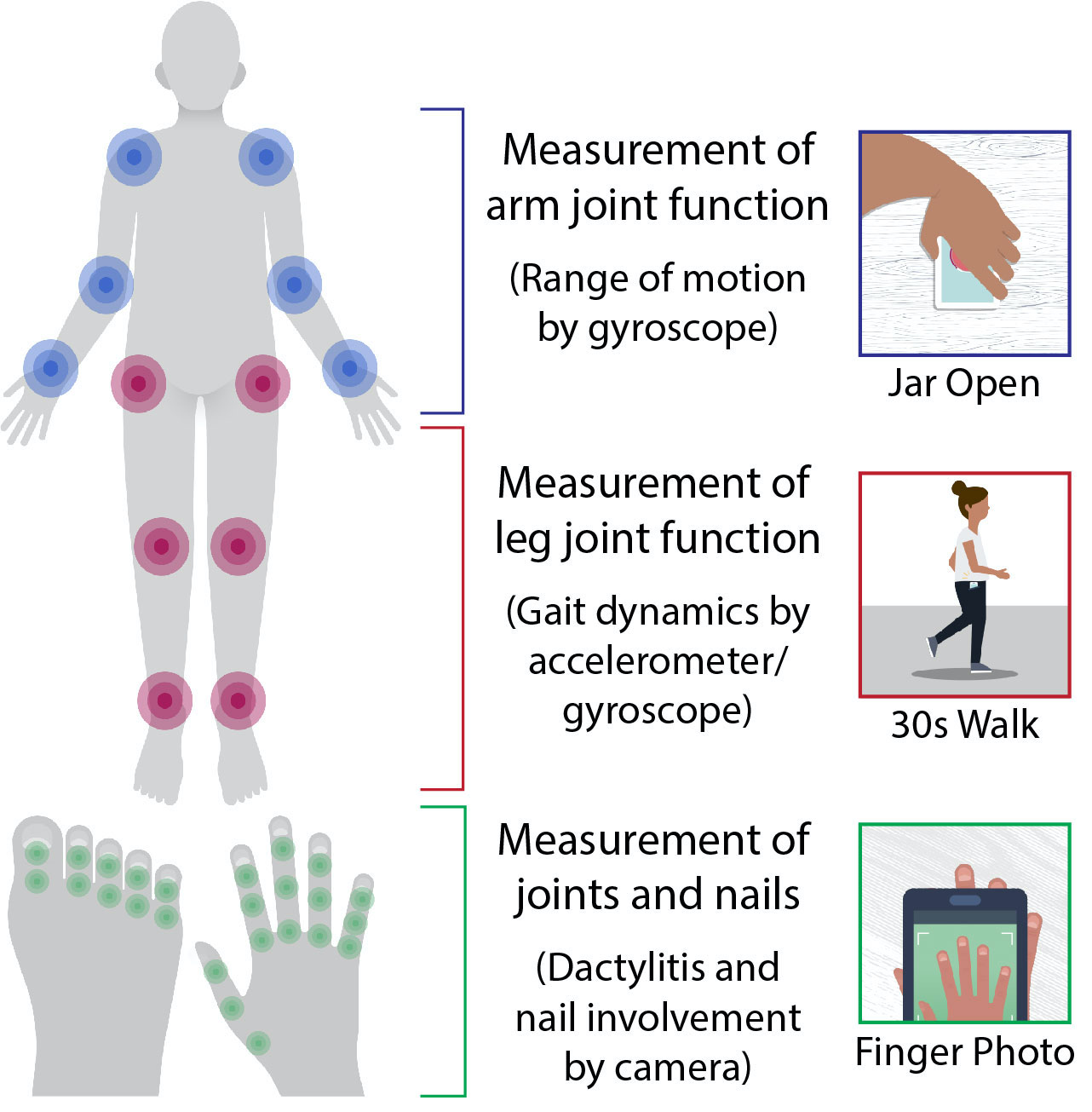 Figure 1. Interactive smartphone sensor-based measures of PsA symptoms from the Psorcast app and the joints that are assessed in each.
Figure 1. Interactive smartphone sensor-based measures of PsA symptoms from the Psorcast app and the joints that are assessed in each.
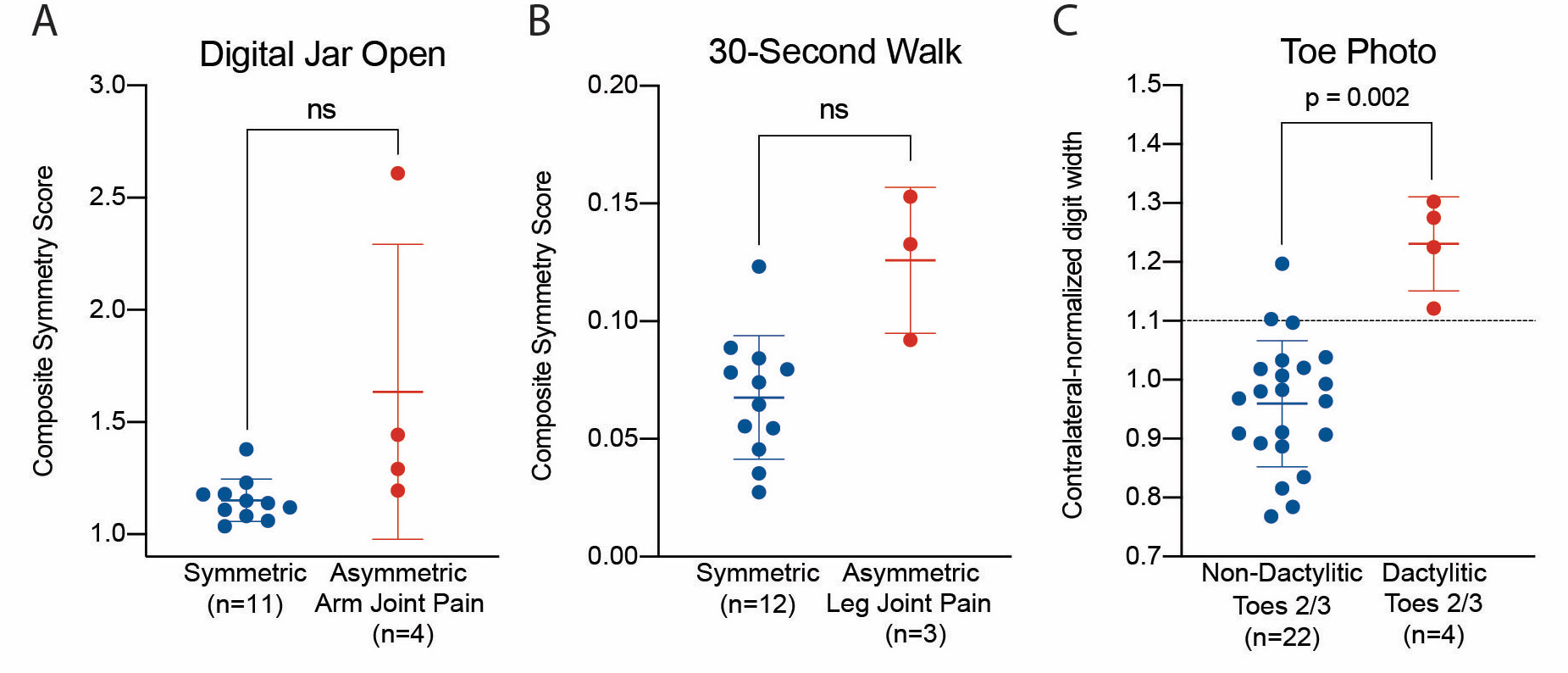 Figure 2. Preliminary results from Psorcast measures. (A) Detection of asymmetric upper extremity joint pain, (B) Detection of asymmetric lower extremity joint pain, (C) Detection of dactylitis in toes 2 and 3. All statistical comparisons use Welch’s t-test.
Figure 2. Preliminary results from Psorcast measures. (A) Detection of asymmetric upper extremity joint pain, (B) Detection of asymmetric lower extremity joint pain, (C) Detection of dactylitis in toes 2 and 3. All statistical comparisons use Welch’s t-test.
To cite this abstract in AMA style:
Webster D, Haberman R, Perez-Chada L, Simon S, MacDuffie W, DePhillips M, Reddy S, Ogdie A, Mangravite L, Merola J, Scher J. Development and Preliminary Validation of Smartphone Sensor-based Measurement Tools for Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/development-and-preliminary-validation-of-smartphone-sensor-based-measurement-tools-for-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-and-preliminary-validation-of-smartphone-sensor-based-measurement-tools-for-psoriatic-arthritis/
