Session Information
Date: Monday, November 13, 2023
Title: Abstracts: Innate Immunity
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Lupus (SLE) is one of the leading causes of death in young women in the United States. Roughly ~80% of SLE patients experience sensitivity to ultraviolet light (UV), which leads to local and systemic inflammation and can trigger nephritis flares. While we previously showed skin exposure to UV leads to neutrophil-mediated kidney damage, the exact mechanisms are unknown. We use scRNA-Seq to characterize kidney injury pathways resulting from skin UV exposure.
Methods: B6 female mice (3-mo) were exposed to UVB (1x500mJ/cm2). On day 2 (D2) or No UV, cardiac perfusion was performed with Dynabeads for glomerular magnetic isolation. Glomeruli were digested (Col IV, Pronase I, DNase I) following Col. I kidney digest and scRNA-seq performed by 10X Genomics. Glomeruli from anti-GCSF and IgG D2 UV-exposed mice were digested and sent for 10X Genomics scRNA-seq. Neutrophils were enriched from digested skin, lungs, and kidney by Ly6G magnetic beads and sorted for >90% purity. Single Cell clustering and differential gene expression (DGE) analyses were performed using Seurat 4.1.1 R package. Gene ontology pathway analysis was performed using clusterProfiler. Ligand-receptor analysis was performed using CellChat. Injury scores created using DGE from D2-UV renal structural cells.
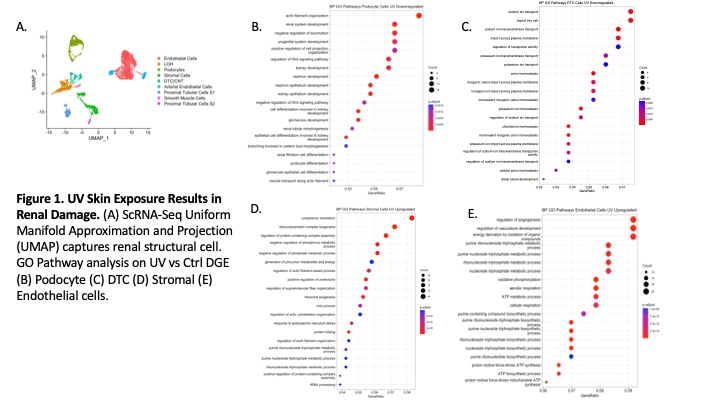
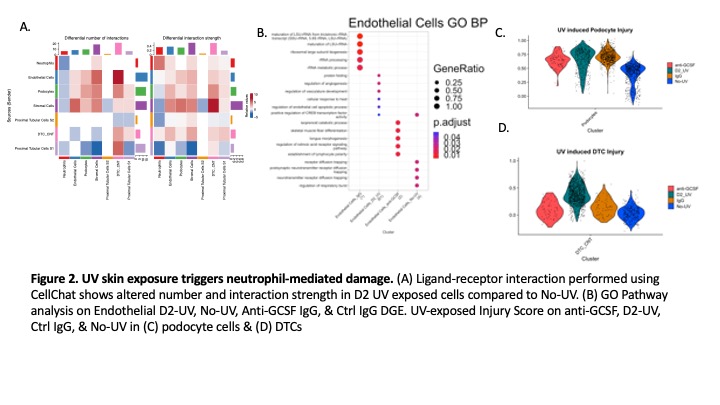
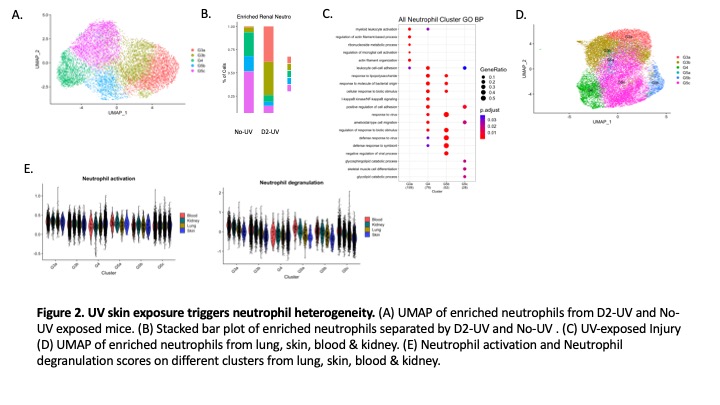
Results: ScRNAseq analysis of kidney structural cells revealed endothelial, stromal, podocyte and distal tubular cell (DTC) injury on D2 after skin UV exposure. Pathway analysis showed downregulation of actin filament organization in podocytes and ion transport in DTCs (Fig 1B-C). Type I interferon pathway was upregulated in stromal cells, while angiogenic and cell stress pathways were increased in renal endothelial cells (Fig 1D-E). Kidney-infiltrating neutrophils showed an increased number and strength of interactions with stromal, endothelial, and DT cells (Fig 2A). Preventing neutrophil recruitment by anti-GCSF IgG abolished endothelial injury pathways (Fig 2B) and decreased injury scores in podocytes and DTCs (Fig2C-D). Five distinct neutrophil clusters (G3a, G3b, G4, G5b, & G5c) were identified in the kidney, though activated immature G3a and G3b neutrophil clusters were only present after UV (Fig3A-B). Pathway analysis showed G3a was enriched for myeloid leukocyte activation and actin regulation pathways (Fig3C). ScRNA-Seq analysis revealed neutrophil heterogeneity at the local site of injury (skin) vs. the distal organs (kidney, lung, & blood) (Fig3D). Kidney infiltrating neutrophils had increased degranulation and activation scores in the kidney vs. the lung or skin (Fig3E).
Conclusion: We identified that neutrophils primarily cause endothelial renal injury but also contribute to podocyte and DTC damage after skin UV exposure. The increased number and strength of podocyte and stromal interactions indicates UV skin exposure triggers injury to the glomerular filtration barrier, supporting our previous findings of UV triggered proteinuria. Altered neutrophil heterogeneity at the local site of injury and distal organs demonstrates tissue specificity in the skin-kidney pathogenic axis.
To cite this abstract in AMA style:
Cortez A, Mendyka L, Kolling F, Whitfield M, Skopelja-Gardner S. Defining Neutrophil-Mediated Renal Damage Triggered by Ultraviolet (UV) Skin Exposure [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/defining-neutrophil-mediated-renal-damage-triggered-by-ultraviolet-uv-skin-exposure/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/defining-neutrophil-mediated-renal-damage-triggered-by-ultraviolet-uv-skin-exposure/