Session Information
Date: Monday, November 13, 2023
Title: (0859–0885) Osteoarthritis & Joint Biology – Basic Science Poster
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Osteoarthritis is associated with bone changes such as subchondral sclerosis, bone marrow lesions, and osteophyte formation (Donel S, 2019). Prior work has demonstrated that CD14-deficient mice show significantly less OA-associated subchondral bone (Sambamurthy N, 2018). CD14 is a GPI-anchored surface protein and co-receptor for several inflammatory TLRs, and is highly expressed in myeloid cells including osteoclast precursors (Zanoni, I 2013; Xue, J 2020). TLR activation can both activate and inhibit osteoclastogenic potential. Therefore, using a CD14-deficient mouse model we hypothesized that inhibitory effects of TLR-stimuli on RANKL-mediated osteoclast differentiation would be ameliorated in CD14 deficient cells.
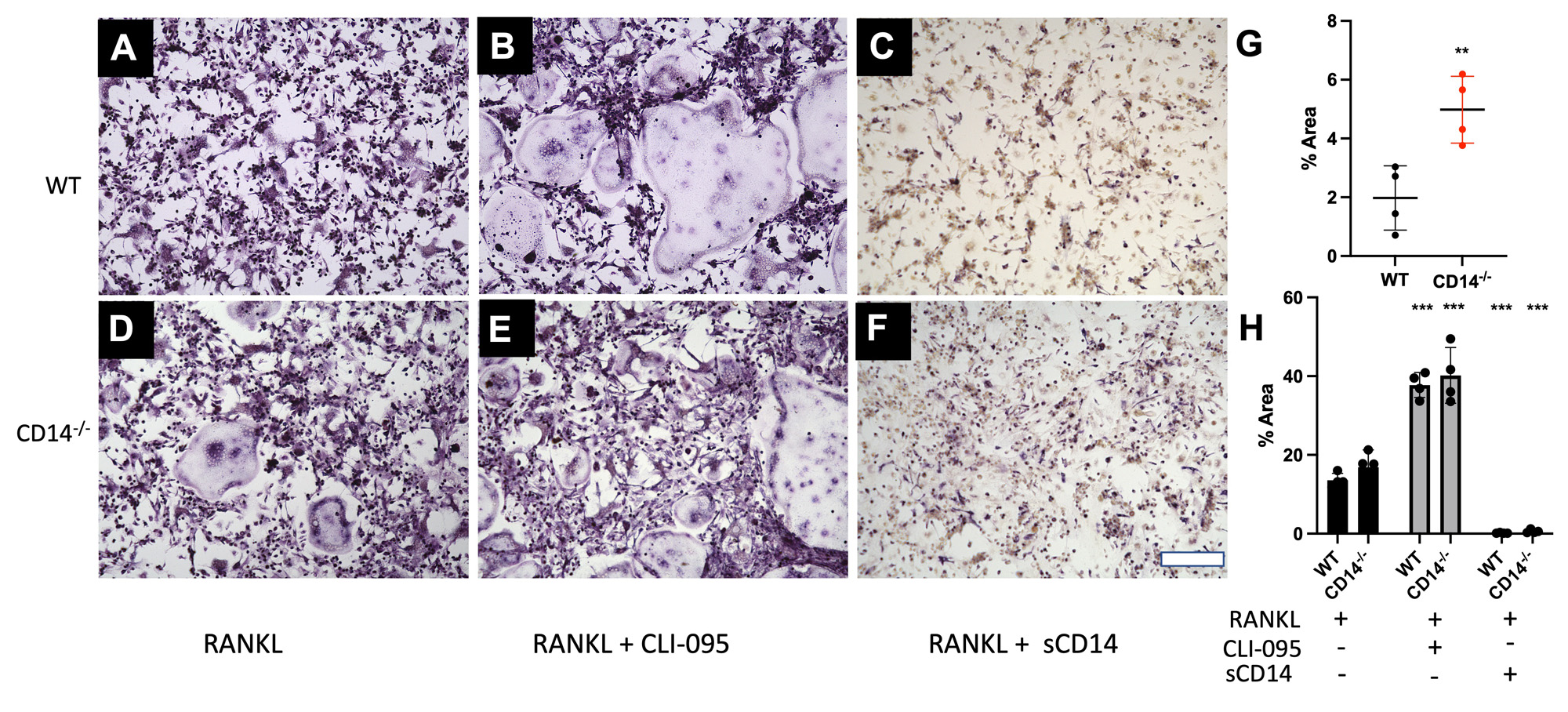
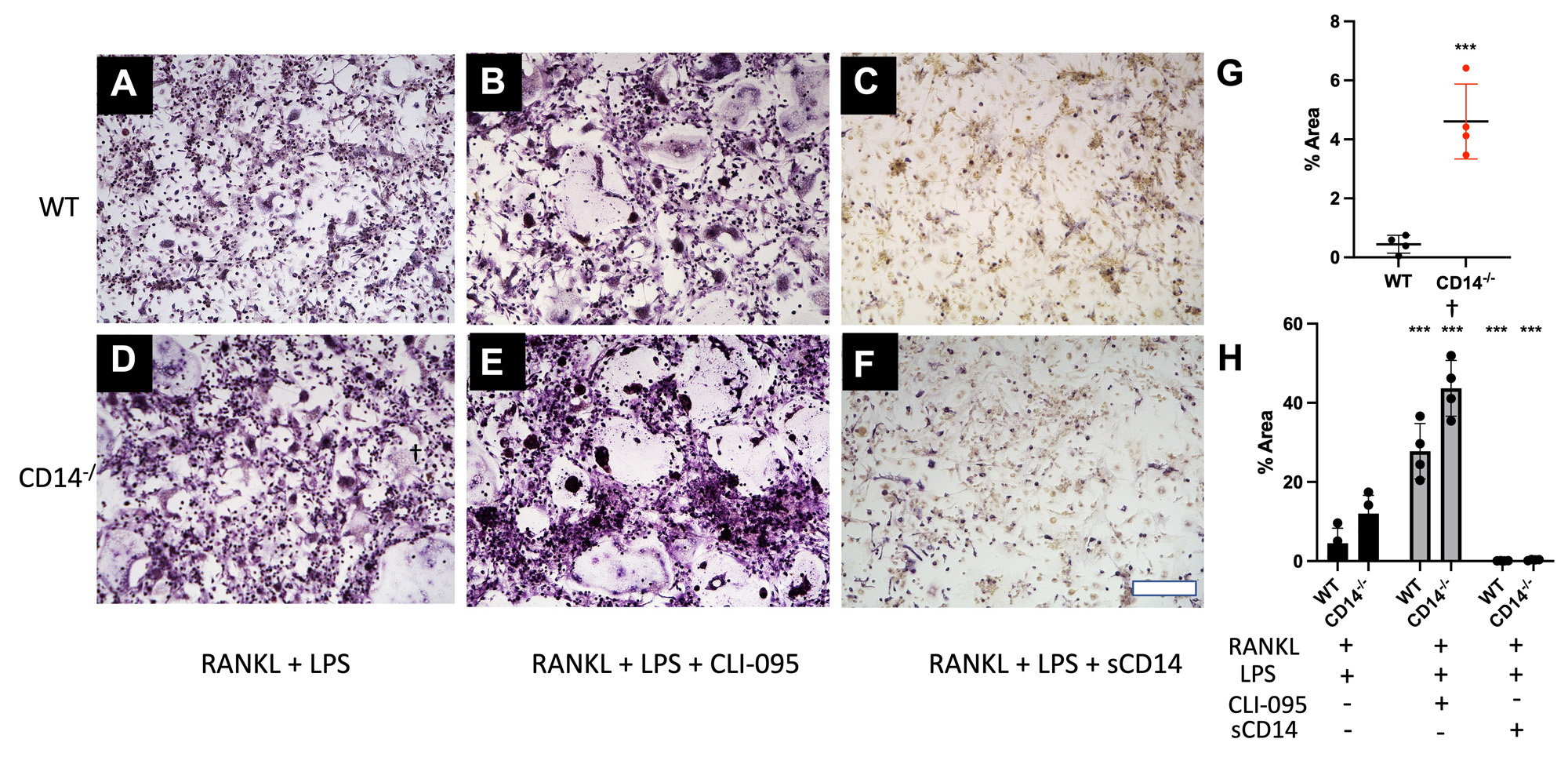
Methods: Cell isolation and culture (n=3-5): Bone Marrow was isolated from femurs and tibiae of skeletally mature (10-12 wk old) C57BL/6 (WT) and CD14-deficient mice. Following 24 hr suspension culture, cells were cultured in complete DMEM with 30 ng/mL M-CSF, for 5 days to expand osteoclast precursors (macrophages). Cells were passaged on day 6 and cultured (24 well plate, 50,000 cells/well) in the presence or absence of RANKL (100 ng/mL). In a separate study, cells were stimulated with a TLR4-stimulus (LPS, 1 ng/mL), soluble CD14 (1 µg/mL), and a TLR4-inhibitor (CLI-095, 1 µg/mL).
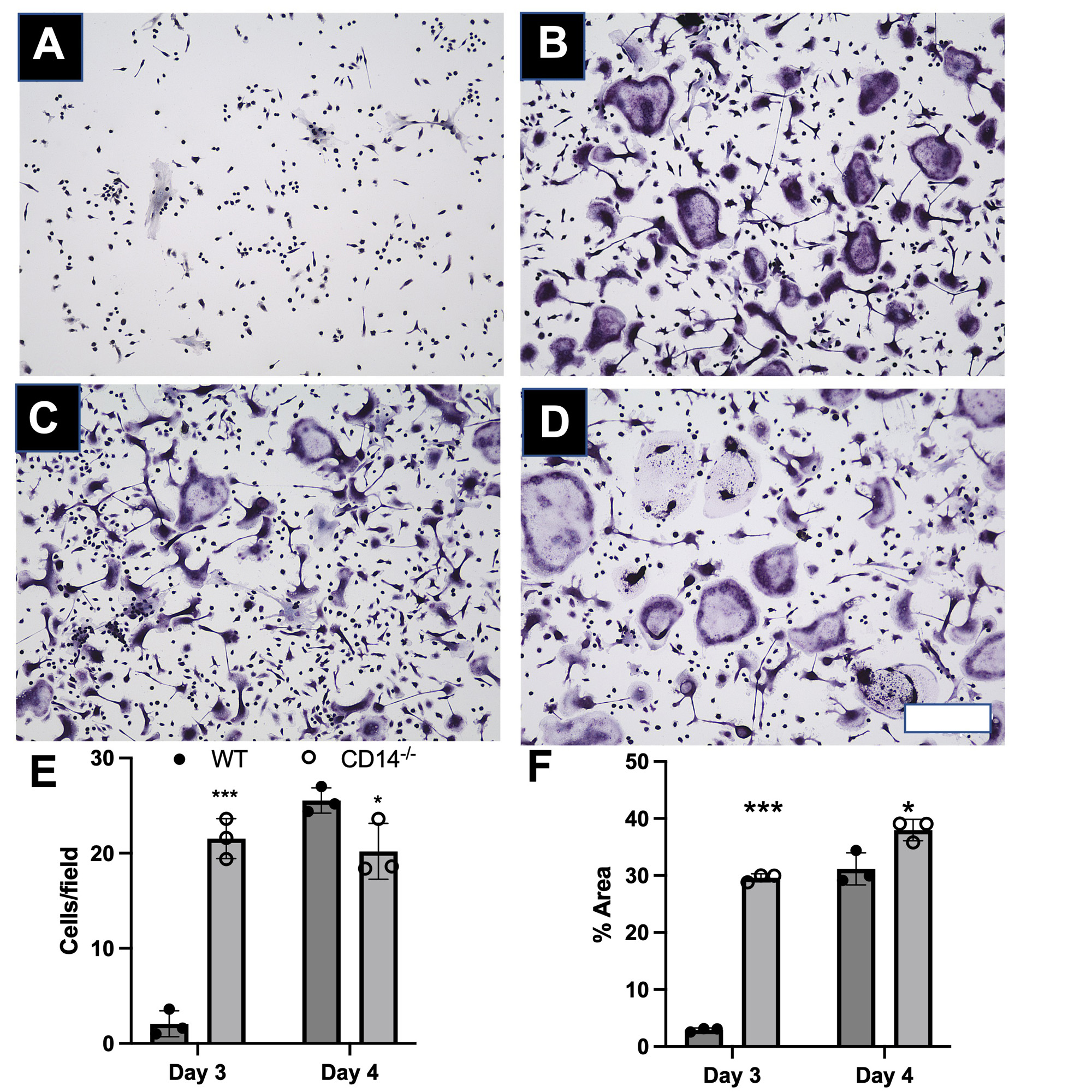
Osteoclast staining and image analysis: Cells were stained for Tartrate-resistant acid phosphatase (TRAP) on days 3 and 4 after addition of RANKL. Cells were imaged at 10x (3 images/well over 4 wells per timepoint) and quantified (% area covered) using ImageJ (NIH) and CellProfiler. Number of osteoclasts (cells with >3 nuclei) per 10X field was also quantitated. Multiple unpaired t-test were performed with Holm-Sidak correction.
Results: CD14-deficient cells showed more rapid differentiation than WT cells at baseline (Fig. 1). Numbers of osteoclasts (Fig. 1E) and area of the plate covered by osteoclasts (Fig. 1F) were higher in CD14-deficient cells on day 3 and day 4 (p< 0.001 and p < 0.05, respectively). TLR4-stimuli: With LPS stimulation, CD14-deficient cells differentiated more quickly compared to WT at day 3 (p< 0.001) (Fig. 3G). LPS stimulation led to a 77% decrease of osteoclastogenesis in the WT cells, but only a 7.5% decrease in osteoclastogenesis on the CD14-deficient cells (Figure 2G, 3G) Addition of CLI-095 mitigated some inhibitory effects of LPS in WT, but had a synergistic effect on CD14-deficient cells (Fig 3H). The addition of sCD14 inhibited osteoclastogenesis in the CD14-deficient group, both with and without LPS.
Conclusion: Our results show that at baseline, CD14-deficient osteoclasts precursors differentiate more quickly than WT cells. Further, during TLR4 stimulation studies, CD14-deficient cells were protected from LPS-TLR4 mediated inhibition of osteoclastogenesis, compared to WT cells. Though mechanism of early differentiation remains unclear, with possible intervention of TLR-ligand production during differentiation, further work will investigate this production, and further effects of OA-relevant TLR ligands on osteoclastogenesis in the setting of CD14 deficiency or blockade.
To cite this abstract in AMA style:
Murphy L, Burt K, Hu B, Nguyen V, Mauck R, Scanzello C. Deficiency of the Pattern-recognition Receptor CD14 Protects Against LPS-induced Inhibition of Osteoclastogenesis in Vitro [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/deficiency-of-the-pattern-recognition-receptor-cd14-protects-against-lps-induced-inhibition-of-osteoclastogenesis-in-vitro/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/deficiency-of-the-pattern-recognition-receptor-cd14-protects-against-lps-induced-inhibition-of-osteoclastogenesis-in-vitro/