Session Information
Date: Sunday, October 26, 2025
Title: Abstracts: Rheumatoid Arthritis – Etiology and Pathogenesis (0795–0800)
Session Type: Abstract Session
Session Time: 1:15PM-1:30PM
Background/Purpose: Rheumatoid arthritis (RA) is driven by complex inflammatory pathways involving immune cell infiltration into synovial tissue and fluid. Synovial fluid (SF) provides direct insight into local disease mechanisms. Few studies have applied multiplex or single-cell transcriptomic technologies to dissect its cytokine and cellular composition. The objective was to (1) characterize the cytokine profile of SF in RA vs other chronic inflammatory rheumatic diseases (CIRD) and osteoarthritis (OA); (2) compare SF and serum cytokines in RA; (3) explore the single-cell transcriptomic signature of RA SF mononuclear cells (SFMCs) vs. peripheral blood mononuclear cells (PBMCs).
Methods: SF samples from 115 patients (45 RA, 48 CIRD, 22 OA) were analyzed using Luminex (21 cytokines including TNF-α, IL-1β, IL-6, IL-17, GM-CSF, IL-23, and CCL20). Paired serum samples were available for 29 RA patients. scRNA-seq was performed on freshly isolated SFMCs and PBMCs from six RA patients using 10X Chromium and CellRanger pipelines. Unsupervised clustering and differential expression analyses were used to define cellular subpopulations and compare their distribution.
Results: RA SF showed significantly higher levels of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-17, Granzyme B, CCL20) than OA (Fig.1A). IL-8, CCL2, and CCL3 were also elevated compared to CIRD. In RA, cytokines were consistently higher in SF than in serum and strongly correlated with CRP and DAS28-CRP, while no significant correlations were observed in serum (Fig. 1B). Heatmap analysis identified two distinct cytokine profiles in RA SF: one characterized by high cytokine secretion and elevated leukocyte count and CRP, and another with lower or moderate cytokine levels, reflecting heterogeneity in local inflammatory responses among RA patients.scRNA-seq identified 27 immune cell clusters: 7 myeloid, 20 lymphoid (Fig. 2A). SFMCs were enriched in conventional (FC = 3.5) and plasmacytoid dendritic cells (FC = 5.5), and depleted in naïve B and T cell subsets, including CD4+ and CD8+ T cells (Fig. 2B). A distinct CD4+ T cell subset expressing PD-1, IL-17, Granzyme A, and IFN-γ was identified in SFMCs, indicating a pro-inflammatory phenotype. Regulatory T cells were enriched, while cytotoxic and effector memory CD8+ T cells were reduced (Fig. 2B).
Conclusion: RA SF exhibits a compartmentalized immune response characterized by cytokine enrichment and effector cell activation. Elevated levels of CCL20, CCL2, CCL3, and CCL8 may support dendritic cell and effector T cell recruitment into the joint. Despite this chemotactic environment, naïve T cells were markedly depleted, suggesting selective homing or local maturation. Increased IL-17 and Granzyme B at both protein and transcript levels were consistent with the presence of an inflammatory CD4+ T cell cluster co-expressing IL17A, GZMA, and PRF1. These findings reveal a coordinated inflammatory network in RA synovium, linking chemokine-mediated recruitment to local T cell activation, and highlight potential therapeutic targets identified through integrated cytokine and single-cell analysis.
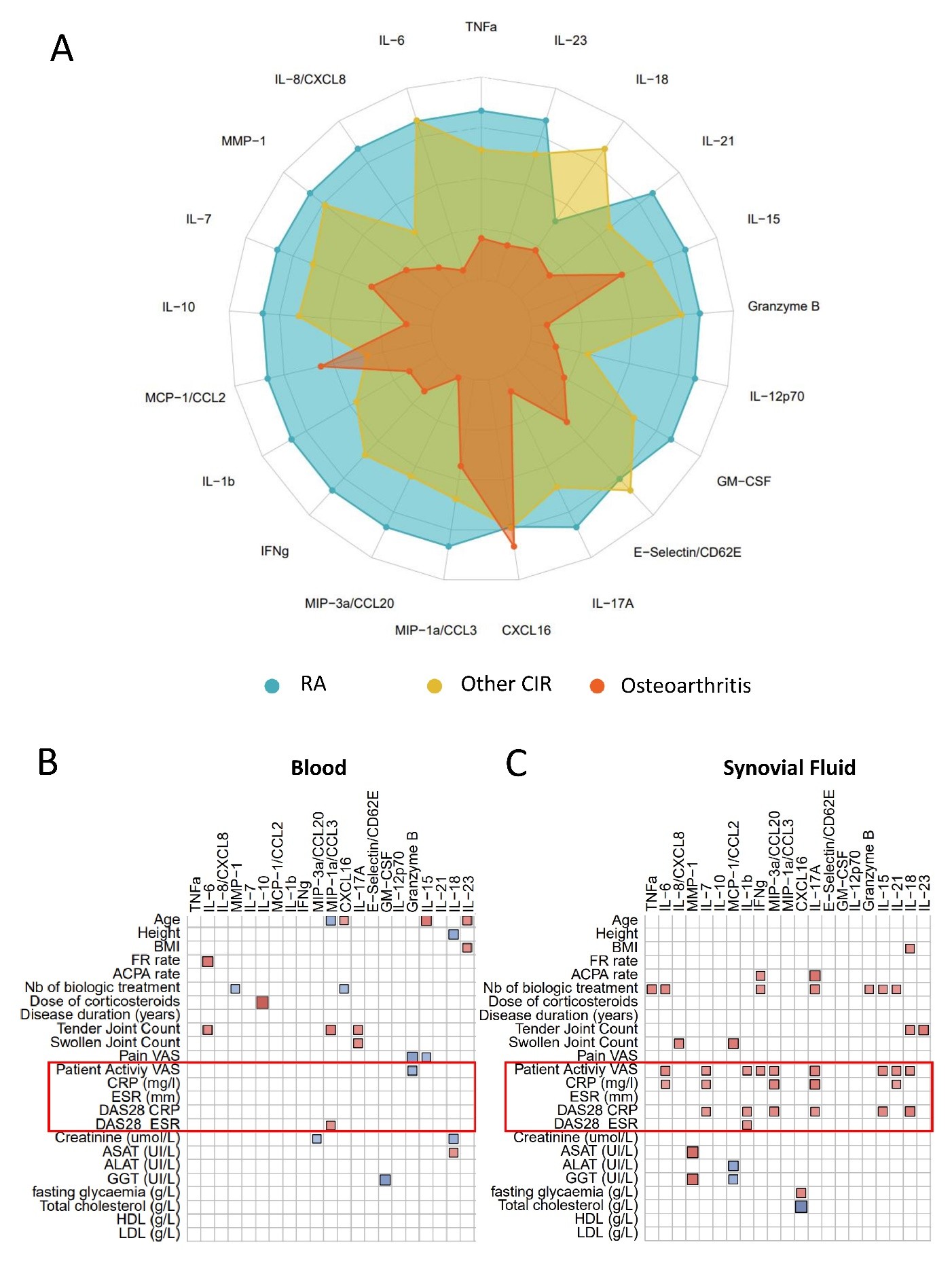 Figure1. Cytokine profiles and correlations with clinical and biological parameters in RA. (A) Radar plot illustrating the relative concentrations of 21 cytokines in synovial fluid (SF) from rheumatoid arthritis (RA, blue), other chronic inflammatory rheumatic diseases (CIR, yellow), and osteoarthritis (OA, orange). Each axis represents a specific cytokine, with values normalized to highlight differences between disease groups. (B) Correlation matrix (corrplot) between cytokines measured in blood and various clinical and biological parameters in RA patients. (C) Correlation matrix (corrplot) between cytokines measured in blood and various clinical and biological parameters in RA patients.
Figure1. Cytokine profiles and correlations with clinical and biological parameters in RA. (A) Radar plot illustrating the relative concentrations of 21 cytokines in synovial fluid (SF) from rheumatoid arthritis (RA, blue), other chronic inflammatory rheumatic diseases (CIR, yellow), and osteoarthritis (OA, orange). Each axis represents a specific cytokine, with values normalized to highlight differences between disease groups. (B) Correlation matrix (corrplot) between cytokines measured in blood and various clinical and biological parameters in RA patients. (C) Correlation matrix (corrplot) between cytokines measured in blood and various clinical and biological parameters in RA patients.
Only significant Spearman correlation coefficients are represented by the intensity of the colour and the size of the square. Red: positive correlation. Blue: negative correlation. The boxed area highlights strong correlations between cytokines and markers of clinical disease activity, including CRP, DAS28-CRP, and patient-reported pain (VAS).
ACPA: Anti-citrullinated protein antibodies. CRP: C-reactive protein. DAS28: Disease Activity Score in 28 joints. ESR: Erythrocyte sedimentation rate. VAS: Visual Analog Scale. TNF-α: Tumor Necrosis Factor-alpha. IL: Interleukin. CXCL/CCL: Chemokines.
.jpg) Figure 2. Immune cell composition in peripheral blood and synovial fluid of rheumatoid arthritis patients (A) Uniform Manifold Approximation and Projection (UMAP) visualization of immune cell populations from peripheral blood mononuclear cells (PBMCs) and synovial fluid mononuclear cells (SFMCs) of patients with rheumatoid arthritis. Distinct cell populations are annotated based on marker expression, including dendritic cells (pDC, cDC), B cells (naive, memory), T cells (CD4-naive, CD8-naive, CD8-effector, CD8-cytotoxic, regulatory T cells, Tregs), MAIT cells, and others. (B) Boxplots showing the proportions of specific immune cell subsets in PBMCs versus SFMCs. Cell subsets analyzed include pDC, cDC, naive B cells, naive CD4 T cells, naive CD8 T cells, CD8 effector memory T cells, CD8 cytotoxic T cells, and regulatory T cells.
Figure 2. Immune cell composition in peripheral blood and synovial fluid of rheumatoid arthritis patients (A) Uniform Manifold Approximation and Projection (UMAP) visualization of immune cell populations from peripheral blood mononuclear cells (PBMCs) and synovial fluid mononuclear cells (SFMCs) of patients with rheumatoid arthritis. Distinct cell populations are annotated based on marker expression, including dendritic cells (pDC, cDC), B cells (naive, memory), T cells (CD4-naive, CD8-naive, CD8-effector, CD8-cytotoxic, regulatory T cells, Tregs), MAIT cells, and others. (B) Boxplots showing the proportions of specific immune cell subsets in PBMCs versus SFMCs. Cell subsets analyzed include pDC, cDC, naive B cells, naive CD4 T cells, naive CD8 T cells, CD8 effector memory T cells, CD8 cytotoxic T cells, and regulatory T cells.
Significant differences between PBMCs and SFMCs are indicated by an asterisk (*).
PBMCs: Peripheral blood mononuclear cells. SFMCs: Synovial fluid mononuclear cells. pDC: Plasmacytoid dendritic cells. cDC: Conventional dendritic cells. Tregs: Regulatory T cells. MAIT: Mucosal-associated invariant T cells. UMAP: Uniform Manifold Approximation and Projection
To cite this abstract in AMA style:
LESTURGIE-TALAREK M, Carbone F, Gonzalez V, Schvartz A, Hecquet S, Oudart F, Thomas M, D'Alessandro R, Allanore Y, Menager M, AVOUAC J. Deciphering Synovial Fluid Immune Dysregulation in Rheumatoid Arthritis through Cytokine Profiling and Single-Cell Transcriptomics [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/deciphering-synovial-fluid-immune-dysregulation-in-rheumatoid-arthritis-through-cytokine-profiling-and-single-cell-transcriptomics/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/deciphering-synovial-fluid-immune-dysregulation-in-rheumatoid-arthritis-through-cytokine-profiling-and-single-cell-transcriptomics/
