Session Information
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Rheumatoid arthritis (RA) and osteoarthritis (OA) are characterized by inflammation, joint swelling, stiffness, and pain. A fibroid phenotype has been identified in RA, where fibrosis is the key molecular feature, and inflammation is absent. Fibroblast-like synoviocytes (FLS) are located in the synovial membrane (SM), where they produce extracellular matrix (ECM) components as a response to injury. The interplay between joint fibrosis and inflammation and its impact on ECM formation are poorly understood. We hypothesize that a fibro-inflammatory environment may affect the fibrotic response of FLS. Our objective was to characterize the fibrotic ECM profile of FLS in a fibro-inflammatory context to gain insights into the complex dynamics of fibrosis and inflammation in joint pathology.
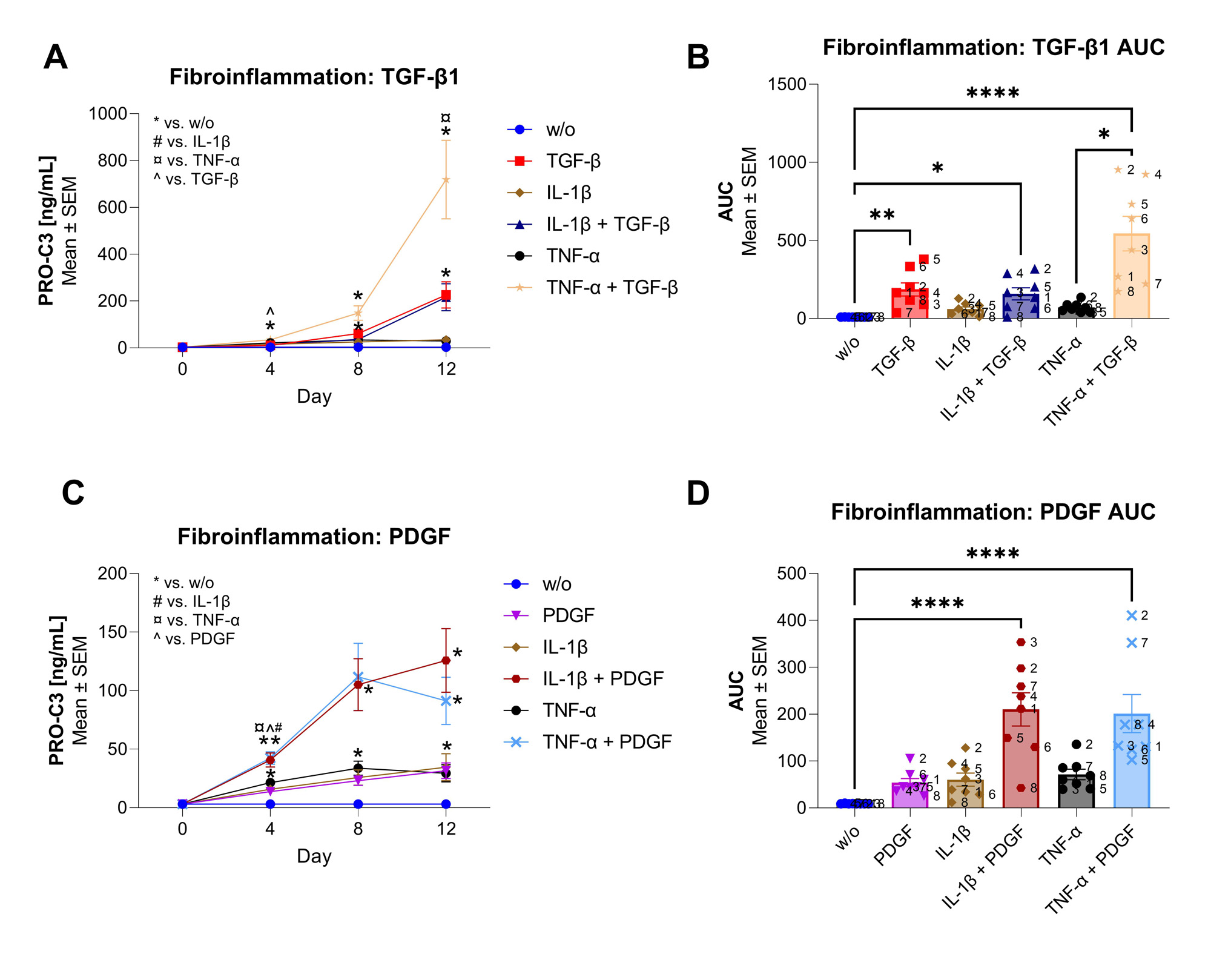
Methods: Primary human FLS were isolated from the SM of patients undergoing total knee replacement surgery (n=8). The FLS were cultured in 0.4% fetal bovine serum DMEM with ficoll (to create a crowded environment) and ascorbic acid for 12 days. The growth factors, TGF-β1 [1 nM] and PDGF-AB [6 nM], and cytokines, IL-1β [0.5 nM] and TNF-α [0.5 nM] were added either alone or in combination. Non-treated FLS were used as control (w/o). Every 4 days, the supernatant was collected, and new treatments were added. PRO-C3, a type III collagen formation biomarker, was measured in the supernatant using a validated ELISA. 2-way ANOVA compared day-to-day differences with Tukey’s multiple comparisons tests. In addition, the AUC for each patient was computed, and the treatment differences were compared using the Kruskal-Wallis test with Dunn’s multiple comparisons tests.
Results: TGF-β increased the PRO-C3 formation from day 8 (D8) and PDGF from D4 compared to w/o (p< 0.05, Fig. 1A, 1B). Co-stimulation with TGF-β+IL-1β increased PRO-C3 on D4 compared to w/o (p< 0.05); however, it was not different from TGF-β or IL-1β alone (Fig. 1A). The AUC of co-stimulation with TGF-β+IL-1β were higher than w/o (p< 0.05, Fig. 1B). In contrast, co-stimulation with TGF-β+TNF-α increased PRO-C3 compared to w/o from D4 and onwards, and were higher than TGF-β on D4 and TNF-α on D12 (p< 0.05, Fig. 1A). The AUC of TGF-β+TNF-α was increased compared to w/o and TNF-α (p< 0.0001 and p< 0.05, respectively, Fig. 1B). Co-stimulation of PDGF+IL-1β, increased PRO-C3 from D4 and onwards compared to w/o, and to PDGF and IL-1β on D4 (p< 0.05, Fig. 1C). The AUC of the co-stimulation was increased compared with w/o (p< 0.0001, Fig. 1D). A similar trend was found for co-stimulation of PDGF+TNF-α. Here, PRO-C3 was increased on D4 and D12 compared to w/o and compared to PDGF and TNF-α on D4 (p< 0.05, Fig. 1C). The AUC of the co-stimulation was higher than w/o (p< 0.0001, Fig. 1D).
Conclusion: In conclusion, the combination of inflammatory cytokines and fibrotic growth factors induces a heightened fibrotic response, particularly increasing type III collagen formation, more than the fibrotic growth factors alone. These data suggest that the coexistence of fibrosis and inflammation could potentially influence the mechanisms driving joint damage in RA and OA. This underscores the potential need for therapeutic strategies targeting both fibrosis and inflammation in patients with arthritis.
To cite this abstract in AMA style:
Madsen S, Andersen M, Scheller Madrid A, Holm Nielsen S, Karsdal M, Thudium C, Bay-Jensen A. Cytokines Regulate the Fibrotic Response of Growth Factors in Joint Fibroblast-like Synoviocytes [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/cytokines-regulate-the-fibrotic-response-of-growth-factors-in-joint-fibroblast-like-synoviocytes/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cytokines-regulate-the-fibrotic-response-of-growth-factors-in-joint-fibroblast-like-synoviocytes/