Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Limited cutaneous systemic sclerosis (lcSSc) is the most frequent subset of scleroderma, yet there is a paucity of outcome measures to assess symptoms that are important to patients with this subset. The CRISTAL project, an international initiative, aims to create a combined response index for lcSSc for use in clinical trials (the CRISTAL index). A key milestone of CRISTAL is the development of a novel patient reported outcome measure (PROM) to assess the most common and/or bothersome symptoms experienced by patients with lcSSc. The objective of this task from the CRISTAL initiative was to create a PROM that assesses the overall symptom experience of lcSSc, following FDA guidance for the creation of PROMs.
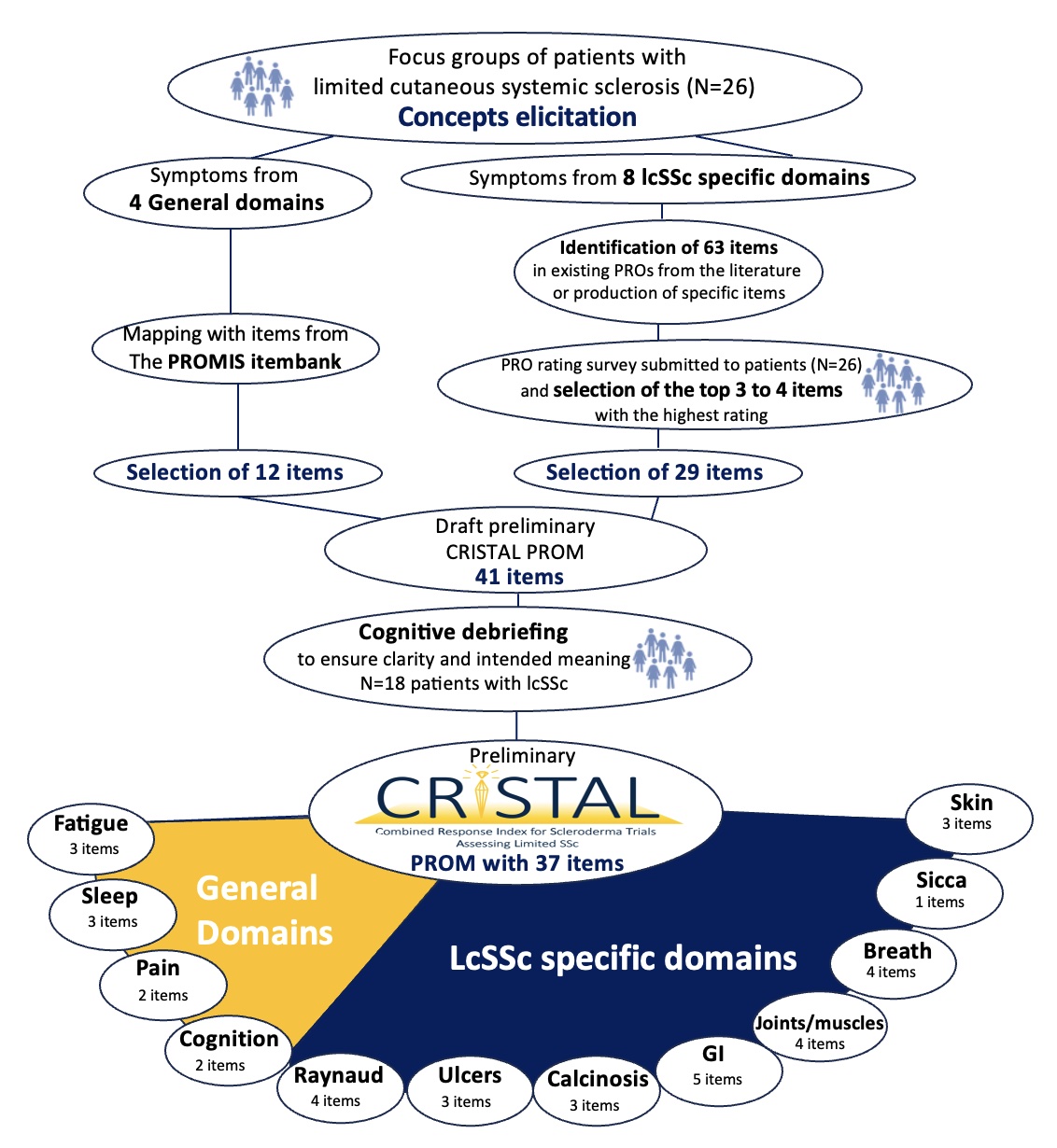
Methods: The CRISTAL PROM was constructed using an iterative approach that included patient partners at each step (Figure 1). 1/Concept elicitation: Face and content validity and domain elicitation were derived through prior focus groups of patients with lcSSc (Lescoat et al. 2022). 2/Item identification and concept mapping: Existing PROM items from PROMIS item banks were mapped to symptoms reflecting general domains (pain, fatigue, cognition, sleep) identified from the focus groups. Existing PROMs from lcSSc-specific domains were identified from the literature. New items were drafted with a patient partner for domains with without existing PROM items (i.e., digital ulcers, calcinosis). 3/Item selection: Items from lcSSc-specific domains were then selected through a patient-centered online survey where patients rated each item on a 5-point scale from “not relevant” to “extremely relevant”. The results informed the development of a draft CRISTAL PROM. 4/Cognitive debriefing: the draft CRISTAL PROM was revised through cognitive interviews with patients with lcSSc to evaluate comprehension and relevance.
Results: 12 general items were selected from PROMIS item banks based on analyses of focus groups. 26 patients with lcSSc were included in the PROM item-rating survey (median disease duration = 4.0 years, 88.5% women); 63 items were rated on relevance. The top 3-4 items with the highest relevance rating per domain were included in the first version of the CRISTAL PROM which included 41 items. This version underwent cognitive debriefing with 18 lcSSc patients (median disease duration 6.0 years, 94.4% women). The CRISTAL PROM V1 was refined through an iterative process based on patients’ insights. The final preliminary CRISTAL PROM includes 37 items assessing symptom experiences reflecting 4 general and 8 lcSSc-specific domains, with a recall period of 7 days.
Conclusion: The preliminary CRISTAL PROM was developed to assess the symptoms that are common and/or bothersome in patients with lcSSc. The next step is to assess the psychometric properties of the CRISTAL PROM in a longitudinal multicenter international cohort of patients with lcSSc.
Acknowledgments: CRISTAL is supported by the World Scleroderma Foundation, Scleroderma & Raynaud’s UK, the Scleroderma Clinical Trial Consortium, and the Scleroderma Research Foundation.
References: Lescoat A, et al. Symptom experience of limited cutaneous systemic sclerosis from the Patients’ perspective: A qualitative study, Semin Arthritis Rheum. 2022
To cite this abstract in AMA style:
Lescoat A, Chen Y, Gedert R, Kortam N, Vann N, Cella D, Shaunfield S, Murphy S, Khanna D. Creation of a New Patient-reported Outcome for a Combined Response Index for Limited Cutaneous SSc Using the FDA Guidance on Patient Reported Outcome Measures: The CRISTAL PRO [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/creation-of-a-new-patient-reported-outcome-for-a-combined-response-index-for-limited-cutaneous-ssc-using-the-fda-guidance-on-patient-reported-outcome-measures-the-cristal-pro/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/creation-of-a-new-patient-reported-outcome-for-a-combined-response-index-for-limited-cutaneous-ssc-using-the-fda-guidance-on-patient-reported-outcome-measures-the-cristal-pro/