Session Information
Date: Saturday, November 16, 2024
Title: Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: CD19 targeting chimeric antigen receptor (CAR) T cells have demonstrated durable drug-free responses and remission in patients with idiopathic inflammatory myopathies (IIM) and systemic lupus erythematosus (SLE) in an academic study series. As a fully human, autologous 4-1BB anti-CD19-CAR T cell therapy, CABA-201 is being evaluated in multiple B cell-mediated autoimmune diseases. RESET-Myositis (NCT06154252) and RESET-SLE (NCT06121297) are ongoing phase I/II clinical trials evaluating the safety & efficacy of CABA-201 in four independent myositis cohorts of immune-mediated necrotizing myopathy (IMNM), antisynthetase syndrome (ASyS), dermatomyositis and juvenile IIM and two separate SLE cohorts of non-renal SLE and lupus nephritis (LN). We report on the initial correlative data of the first 12 weeks & 28 days post- CABA-201 treatment in the first IMNM and the first SLE patient, respectively.
Methods: Sera and PBMCs were collected from SLE & IIM subjects before & after CABA-201 infusion. Sera were evaluated for cytokines by electro-chemiluminescence immunoassay. Sera were also evaluated for IIM, SLE, & pathogen associated antibodies via immunoassay. Flow cytometric analyses were performed on CART and B cells. CART cells were quantified post-infusion via dPCR. Cytolytic analyses of the CABA-201 infusion product were performed via IncuCyte assay.
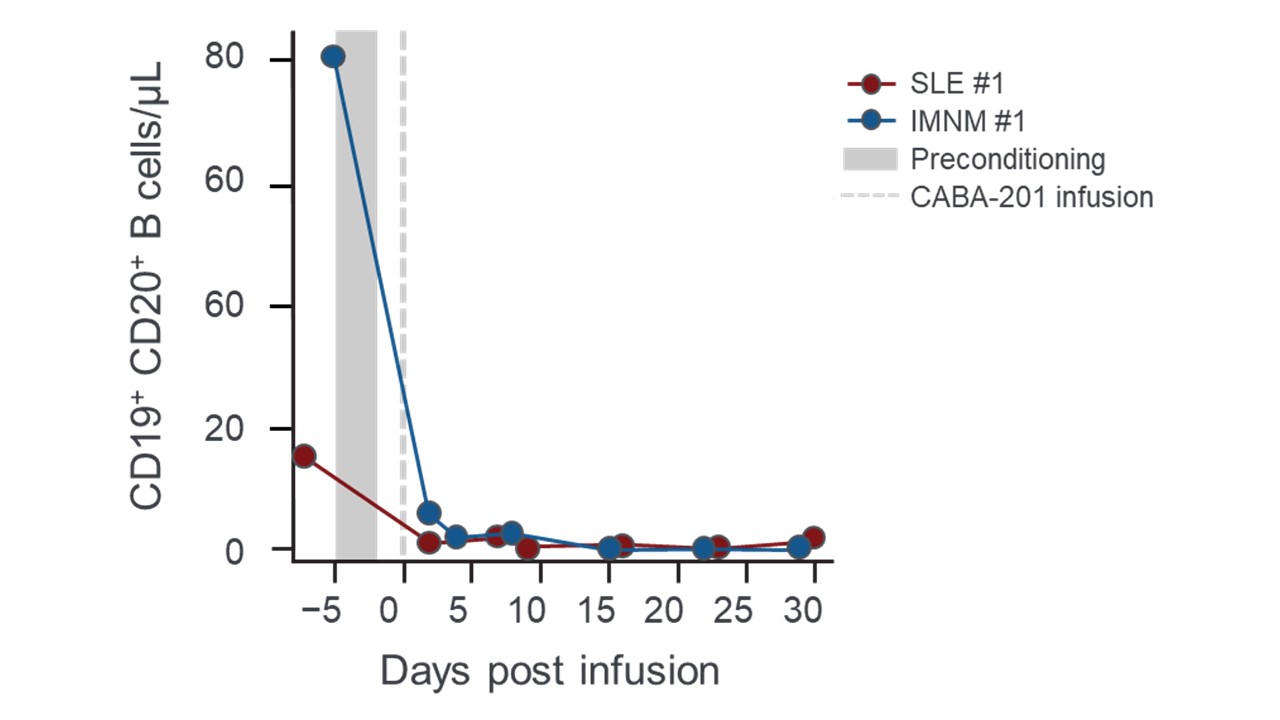
Results: Both patients tolerated CABA-201 treatment with no Serious Adverse Events (SAE), Cytokine Release Syndrome (CRS), or Immune effector cell-associated neurotoxicity syndrome. The CABA-201 infusion product from both patients consisted of predominantly CD4+ effector memory CAR T cells and exhibited in vitro cytolytic activity towards a NALM6 target cell line. Post-infusion, CABA-201 expansion peaked at day 15 in both patients, which was preceded by a serum IFN-γ peak on day 5 and day 8 for the SLE and the IMNM patient, respectively. Leukocyte and lymphocyte counts dropped quickly and transiently after a standard preconditioning regimen of fludarabine (25mg/m2/dy x 3) and cyclophosphamide (1000mg/m2) which inversely corresponded with a transient peak in serum IL-15 levels in both patients. Peripheral B cells in the IMNM patient were predominantly naïve (CD38MidCD24Mid) and transitional (CD38HiCD24Hi) in phenotype at baseline, with a small percentage of memory B cells (CD38NegCD24Hi). B cells in both patients were reduced rapidly following infusion, and full B cell depletion was achieved at day 15 post-infusion for both patients (Figure). Serum levels of B cell activating factor (BAFF) increased throughout the first month in response to B cell depletion. In the IMNM patient, peripheral B cells repopulated by week 8 and were a majority (over 90%) transitional in phenotype with no memory B cells detected. Antibodies associated with infectious diseases and standard vaccinations remained stable in both patients whereas serum levels of myositis-specific and associated autoantibodies decreased in the IMNM patient over the first three months.
Conclusion: These emerging data detail the pharmacokinetics and pharmacodynamics of CABA-201 in IIM and SLE. Evaluation of the selected CABA-201 dose continues in the ongoing RESET-Myositis and RESET-SLE trials.
To cite this abstract in AMA style:
Nunez D, Volkov J, Stadanlick J, Vorndran Z, Ellis A, Werner M, Cicarelli J, Williams J, Nezhad F, Furmanak T, Lam Q, Estremera R, White Y, Hogan J, Miller C, Mozaffar T, Sheikh S, Chang D, Basu S. Correlative Studies of CABA-201, a Fully Human, Autologous 4-1BB Anti-CD19 CAR T Cell Therapy in Patients with Immune-Mediated Necrotizing Myopathy and Systemic Lupus Erythematosus from the RESET-MyositisTM and RESET-SLETM Clinical Trials [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/correlative-studies-of-caba-201-a-fully-human-autologous-4-1bb-anti-cd19-car-t-cell-therapy-in-patients-with-immune-mediated-necrotizing-myopathy-and-systemic-lupus-erythematosus-from-the-reset-myos/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/correlative-studies-of-caba-201-a-fully-human-autologous-4-1bb-anti-cd19-car-t-cell-therapy-in-patients-with-immune-mediated-necrotizing-myopathy-and-systemic-lupus-erythematosus-from-the-reset-myos/