Session Information
Date: Saturday, November 16, 2024
Title: B Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic sclerosis (SSc) affects the skin and internal organs with a poor prognosis. While the exact cause remains unclear, increasing evidence indicates that B cells play a significant role in the pathogenesis by producing autoantibodies, secreting unique cytokines, and activating other immune cells. Rituximab (RTX), a chimeric monoclonal antibody that targets the B-cell specific antigen CD20, has shown efficacy in treating SSc. The DESIRES trial (NCT04274257) demonstrated RTX’s superiority over placebo in reducing the modified Rodnan skin score (mRSS) 24 weeks post-treatment, with a noted association between decreased serum immunoglobulin levels and RTX responsiveness. However, this decrease could not be explained by changes in SSc-related autoantibodies like anti-topoisomerase I antibody (ATA), anti-centromere antibody (ACA), or anti-RNA polymerase III antibody (ARA), prompting further investigation.
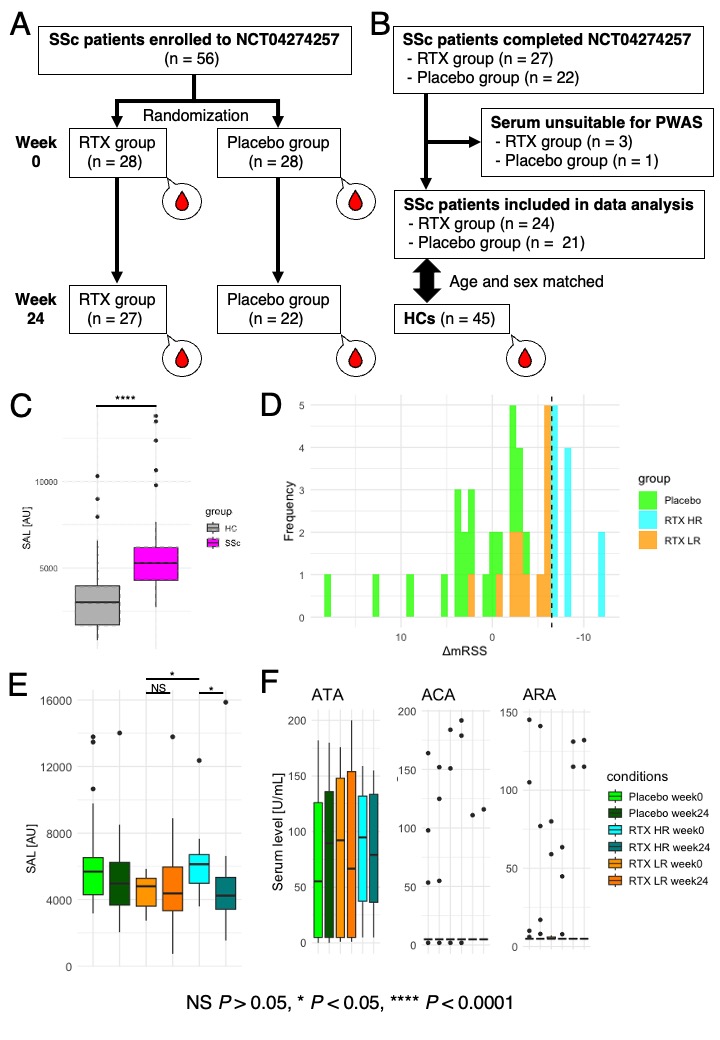
Methods: In the DESIRES trial, 56 SSc patients were evenly randomized to receive RTX or placebo. Serum samples collected at the start and 24 weeks post-treatment were used for proteome-wide autoantibody screening (PWAS; Figure 1A). PWAS was conducted using wet protein arrays that display 13,350 human antigens. After excluding unsuitable samples, the study proceeded with 24 patients in the RTX group, 21 in the placebo group, and 45 sex and age-matched healthy controls (HCs; Figure 1B). Patients with mRSS improvement of 7 or higher and 6 or lower 24 weeks post-treatment were classified as high responders (HRs) and low responders (LRs), respectively. Clinically relevant autoantibodies were defined as those serum levels differentially elevated in SSc compared to HCs, higher in HRs than in LRs at baseline, and significantly reduced pre- and post-RTX administration in HRs.
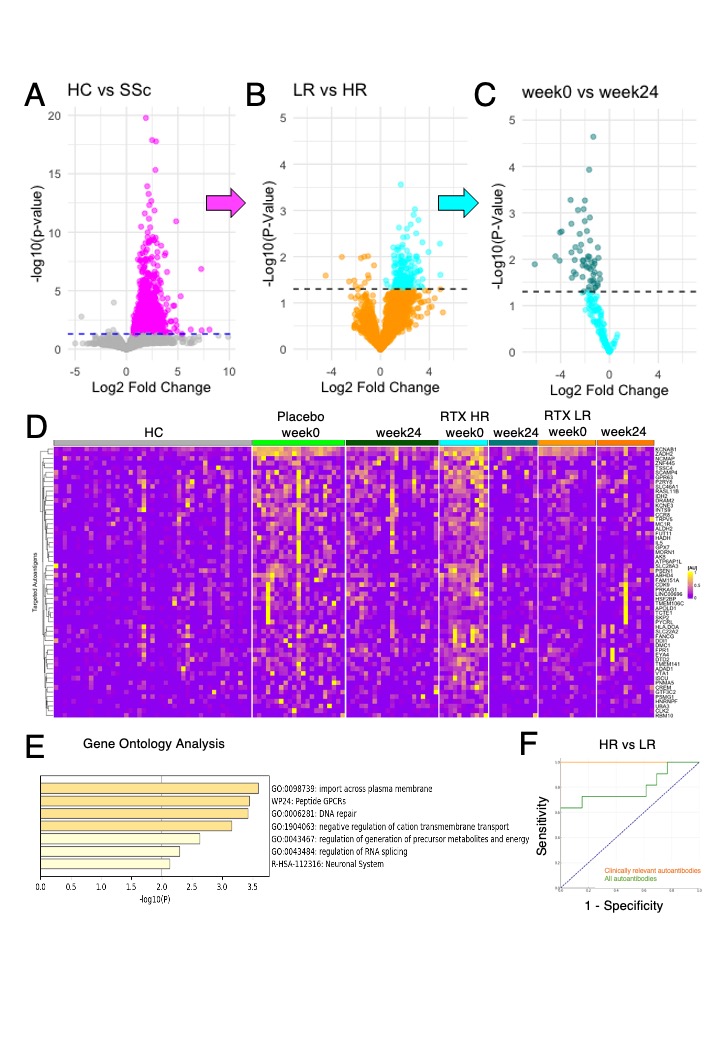
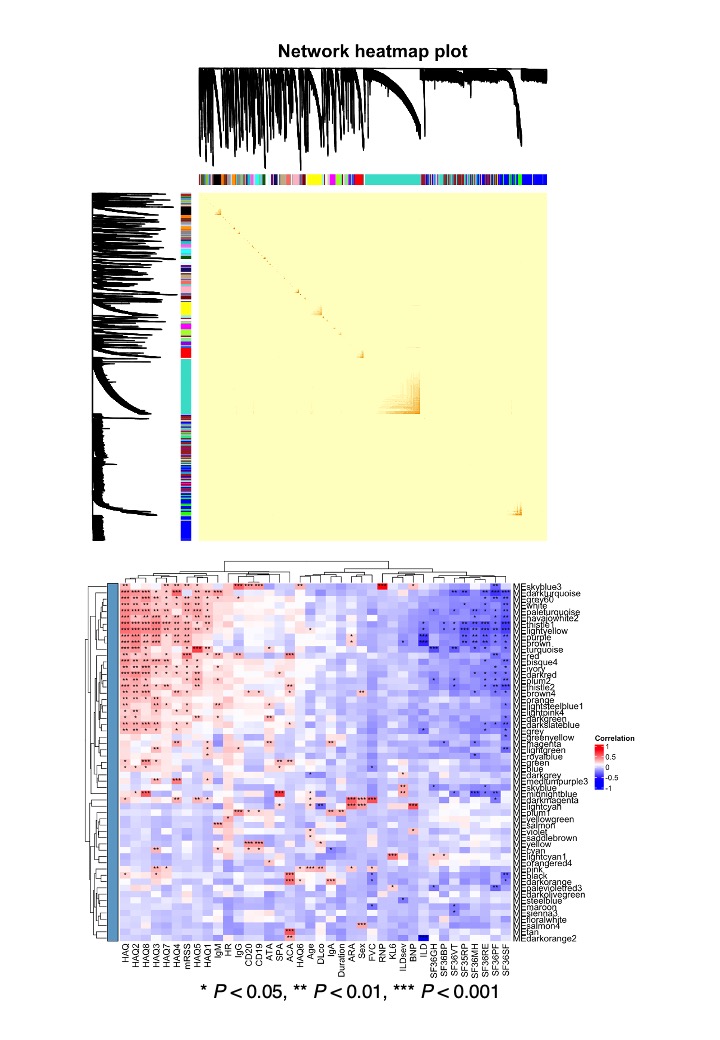
Results: Sum of autoantibody levels (SAL) was significantly higher in SSc patients compared to HCs (Figure 1C). RTX treatment group was categorized into HRs (n = 11) and LRs (n = 13; Figure 1D). HRs showed greater initial SAL and significant reductions post-treatment, unlike LRs (Figure 1E). No such trend was observed in SSc-related autoantibodies, including ATA, ACA, and ARA (Figure 1F). We identified 58 clinically relevant autoantibodies (Figure 2A, B, C, and D), primarily targeting membrane proteins, including G protein-coupled receptors (Figure 2E), which provided near-perfect differentiation between HRs and LRs (Figure 2F). Weighted correlation network analysis identified clustering of most clinically relevant autoantibodies with ATA in the “turquoise” module, correlating with clinical traits of SSc, including mRSS and patient-reported quality of life (Figure 3).
Conclusion: This study applied PWAS to serum samples collected in the DESIRES trial, revealing a significant elevation of autoantibody levels in SSc compared to HCs. We identified 58 clinically relevant autoantibodies responsive to RTX therapy. These findings underscore the potential of comprehensive autoantibody profiling in enhancing diagnostic and therapeutic strategies for SSc, and in deciphering the pathogenesis of SSc.
To cite this abstract in AMA style:
Matsuda K, Sato S, Yoshizaki A. Comprehensive Autoantibody Profiling Highlights Clinical Relevance of Autoantibodies to G Protein-coupled Receptors in Systemic Sclerosis: Insights from a B-cell Depletion Clinical Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/comprehensive-autoantibody-profiling-highlights-clinical-relevance-of-autoantibodies-to-g-protein-coupled-receptors-in-systemic-sclerosis-insights-from-a-b-cell-depletion-clinical-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comprehensive-autoantibody-profiling-highlights-clinical-relevance-of-autoantibodies-to-g-protein-coupled-receptors-in-systemic-sclerosis-insights-from-a-b-cell-depletion-clinical-trial/