Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Mitochondria are extruded upon cell death and platelet activation, being elevated in several inflammatory conditions, including systemic lupus erythematosus (SLE). Mitochondria are immunogenic, with SLE patients having anti-mitochondrial antibodies (AMA), including anti-cardiolipin antibodies, which are associated with thrombosis. However, the role of AMAs, as well as other opsonins such as complement, in regulating mitochondrial-mediated platelet activation, has not been carefully investigated. In the current study, we set out to determine the role of AMAs and complement components in modifying the ability of extracellular mitochondria to promote platelet activation.
Methods: Ultra-pure mitochondria were isolated from HepG2 cells through Dounce homogenization and differential centrifugation. Mitochondria were pre-opsonized with isolated IgG from SLE patients or healthy controls, as well as complement C3-deficient or sufficient plasma, prior to adding the washed opsonized mitochondria to platelets from healthy individuals. Markers of platelet activation were assessed by flow cytometry (P-Selectin; CD62P) or by ELISA of the supernatant (platelet factor 4; PF-4, and thrombospondin-1; TSP-1).
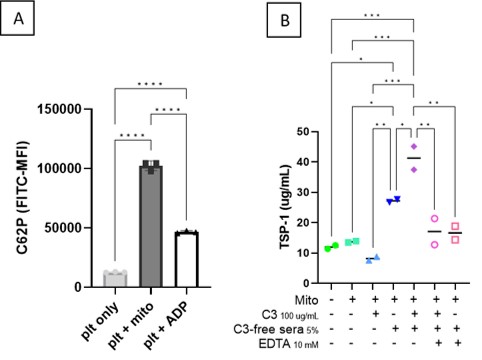
Results: Extracellular mitochondria were able to induce CD62P on the platelet surface (8x increase from baseline, p< 0.0001, Figure 1A), even to a higher extent than ADP, a known platelet activator, used as positive control. Surprisingly, opsonization of mitochondria with SLE IgG only marginally amplified platelet activation. Mitochondria supported complement activation, with binding of both C1q and activated C3 to the outer membrane. Importantly, C3-sufficient, but not deficient, sera supported enhanced mitochondrial-mediated platelet activation as determined by CD62P, PF-4 (3x increase from baseline, p< 0.001) and TSP-1 levels (4x increase from baseline, p< 0.001, Figure 1B). Of note, EDTA, a chelator of divalent cations, Ca2+ and Mg2+ necessary for complement activation, prevented mitochondrial opsonization with C3, and prevented subsequent platelet activation.
Conclusion: Extracellular mitochondria were able to induce CD62P on the platelet surface (8x increase from baseline, p< 0.0001, Figure 1A), even to a higher extent than ADP, a known platelet activator, used as positive control. Surprisingly, opsonization of mitochondria with SLE IgG only marginally amplified platelet activation. Mitochondria supported complement activation, with binding of both C1q and activated C3 to the outer membrane. Importantly, C3-sufficient, but not deficient, sera supported enhanced mitochondrial-mediated platelet activation as determined by CD62P, PF-4 (3x increase from baseline, p< 0.001) and TSP-1 levels (4x increase from baseline, p< 0.001, Figure 1B). Of note, EDTA, a chelator of divalent cations, Ca2+ and Mg2+ necessary for complement activation, prevented mitochondrial opsonization with C3, and prevented subsequent platelet activation.
To cite this abstract in AMA style:
Barguil Macedo M, Lood C. Complement Deposition on Extracellular Mitochondria Induces Platelet Activation in Vitro [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/complement-deposition-on-extracellular-mitochondria-induces-platelet-activation-in-vitro/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/complement-deposition-on-extracellular-mitochondria-induces-platelet-activation-in-vitro/