Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Raynaud’s phenomenon (RP) is the most common symptom in systemic sclerosis (SSc) and can lead to significant morbidity, including digital ulcers and/or gangrene. Despite biologic plausibility and clinical experience supporting the efficacy of several drug classes, clinical trials have demonstrated mixed results. Thus, there are no FDA-approved medications to treat RP. A proposed reason for the inconsistent trial results is the lack of an accurate RP outcome measure. In this study, we compare the traditional paper diary method for recording frequency and duration of RP attacks to a new smartphone application (Figure 1).
Methods: We conducted a multi-center, prospective, cross-over study of patients with stable SSc-RP. Participants were randomized to record their RP attack frequency and duration via paper diary or smartphone for one week and then crossed over to the other recording method the subsequent week. Participants filled out a questionnaire about their experience. We compared patient preferences and experiences, and RP attack frequency and duration by recording method. Comparisons were made by paired t-test, McNemar’s test and Wilcoxon rank sum test as appropriate.
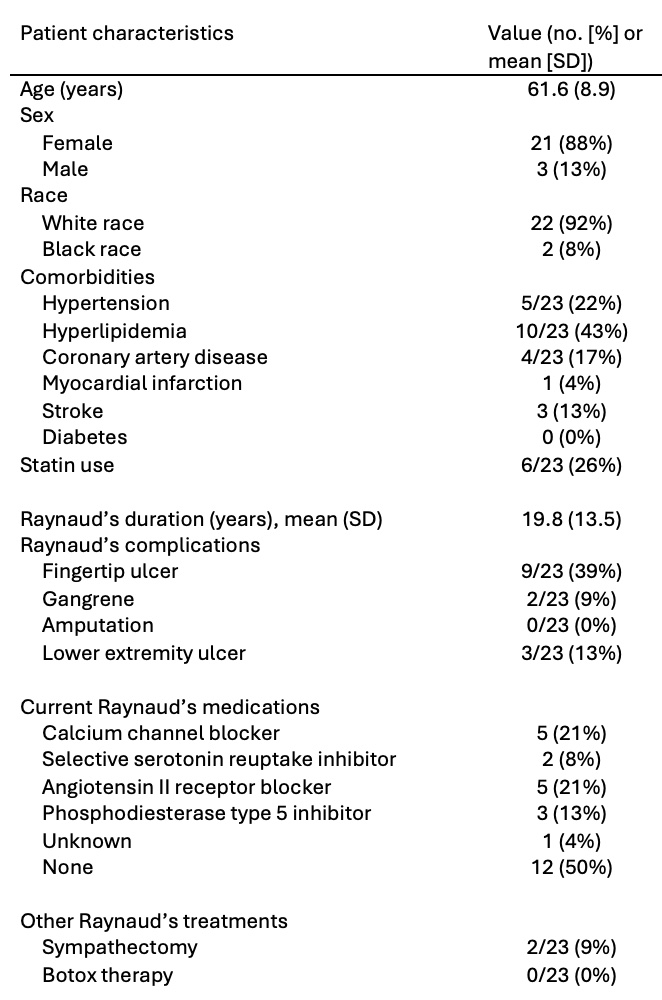
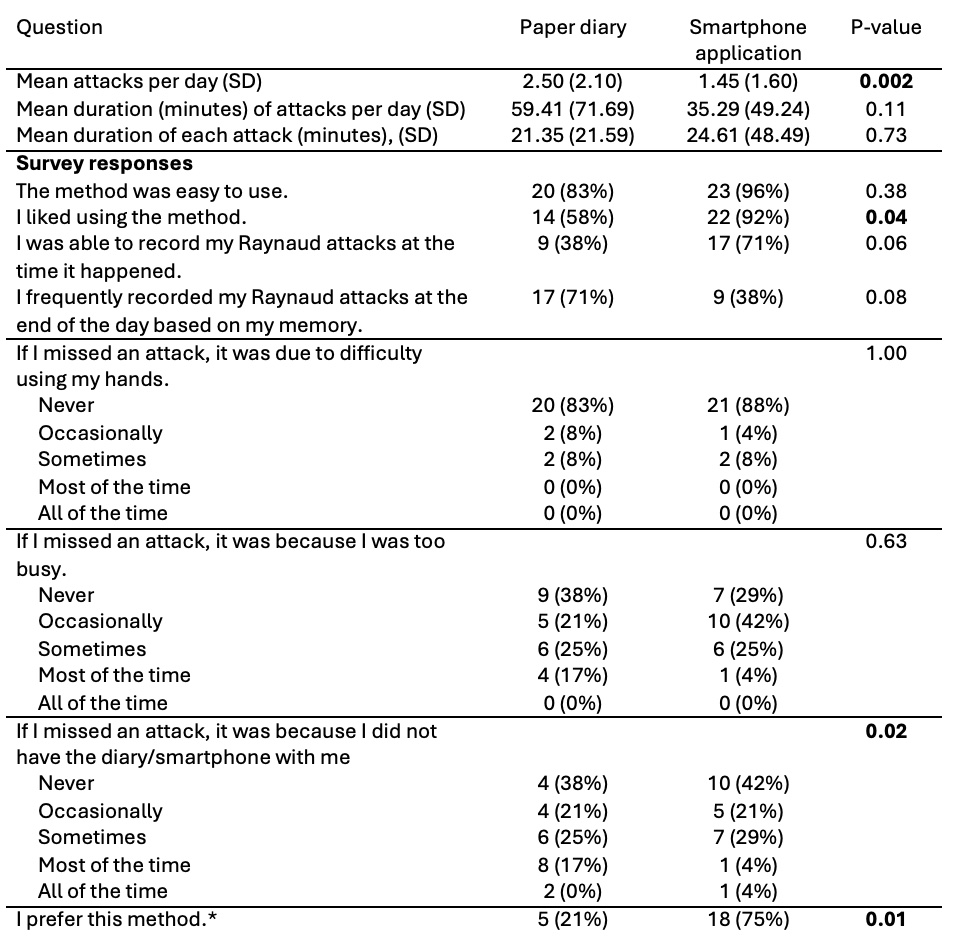
Results: Twenty-four patients with stable SSc-RP completed the study (Table 1). Most participants were female (21 [88%]) with a mean (SD) age of 61.6 (8.9) years and RP duration of 19.8 (13.5) years. Participants felt that both the paper (83%) and smartphone (96%) diary were easy to use (p=0.38). Significantly more reported “liking” the smartphone application than the paper diary (92% vs. 58%, p=0.04). Additionally, 18 (75%) participants preferred the smartphone application, while only 5 (21%) preferred the paper diary and 1 (4%) had no preference (p=0.01).
Importantly, participants more frequently recorded the RP attack in real-time with the smartphone than the paper diary (71% vs. 38%, p=0.06), and participants less frequently recorded RP attacks at the end of the day (based on memory) with the smartphone (38%) vs paper (71%, p=0.08). Participants recorded significantly more attacks with the paper diary than with the smartphone application (mean [SD] attacks/day 2.50 [2.10] vs. 1.45 [1.60], respectively, p=0.002); Table 2). Notably, the average attack length was not significantly different between recording methods (paper diary: 21.35 [21.59] minutes/attack vs. smartphone: 24.61 [48.49] minutes/attack; p=0.73). Participants were more likely to miss an attack because the paper diary was not with them compared with the smartphone application (p=0.02).
Conclusion: This study indicates that patients prefer using a smartphone application compared with a paper diary to record RP attacks. Additionally, since 33% more patients recorded their RP attacks in real-time with the smartphone application, this method is likely more accurate as it is less susceptible to recall bias. The significantly higher number of attacks recorded in the paper diary compared to the smartphone application suggests patients may overestimate their RP attack frequency when completing the form at the end of the day. Future clinical trials should consider using a smartphone-based application to capture RP attacks.
To cite this abstract in AMA style:
Wallwork R, Hu H, Shah A, Hummers L, Pauling J, Flower V, Parmanto B, Saptono A, Domsic R. Comparing Raynaud’s Phenomenon Measurement Tools: Results of the Optimizing Raynaud Phenomenon Outcome Measures in Systemic Sclerosis (ROSS) Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/comparing-raynauds-phenomenon-measurement-tools-results-of-the-optimizing-raynaud-phenomenon-outcome-measures-in-systemic-sclerosis-ross-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparing-raynauds-phenomenon-measurement-tools-results-of-the-optimizing-raynaud-phenomenon-outcome-measures-in-systemic-sclerosis-ross-study/