Session Information
Date: Tuesday, October 28, 2025
Title: (2470–2503) Systemic Sclerosis & Related Disorders – Clinical Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Evaluating disease modification in systemic sclerosis (SSc) is challenging and requires long term studies. This is especially relevant to limited cutaneous (lc)SSc where severe organ involvement occurs later and less frequently than in diffuse cutaneous (dc)SSc. We have tested a novel composite “time to clinically meaningful progression” endpoint (termed MINIMISE) in a large cohort of SSc to evaluate potential as a clinical trial endpoint for long term interventional or observational research across SSc subsets. We have also explored differences in long term outcome between ACA positive and ACA negative lcSSc, reasoning that ACA negative may show greater progression, and be especially informative in clinical trials.
Methods: Demographic, clinical, and serological data were explored in 2894 well characterized patients followed in our centre. We tested performance of the MINIMISE endpoint that assessed time to first development of new diagnosis of pulmonary arterial hypertension at right heart catheterization (according to current haemodynamic definition at time of diagnosis), new evidence of interstitial lung disease (ILD) with forced vital capacity (FVC) < 70% predicted or worsening of established ILD (reduction in FVC ≥10% in 12 months or between 5 and 9% with contemporary reduction in DLCO ≥15%), new onset severe cardiac involvement attributed to SSc (systolic ejection fraction drop to < 45% or pericardial effusion impairing cardiac function or arrhythmia requiring antiarrhythmic therapy or need for a cardiac device), new onset severe gastrointestinal (GI) involvement (enteral nutrition supplementation for ≥3 weeks or any parenteral feeding or hospital admission for intestinal pseudo-obstruction or obstruction), new onset scleroderma renal crisis, new onset severe digital vasculopathy requiring hospitalization, absolute increase of ≥5 modified Rodnan skin score units, or all-cause mortality.We performed a time to event analysis for the MINIMISE composite outcome from disease onset, defined by first non-Raynaud SSc manifestation. Kaplan Meier survival failure estimates curves and Cox proportional hazards regression analysis compared disease and ACA subsets. P< 0.05 were considered statistically significant.
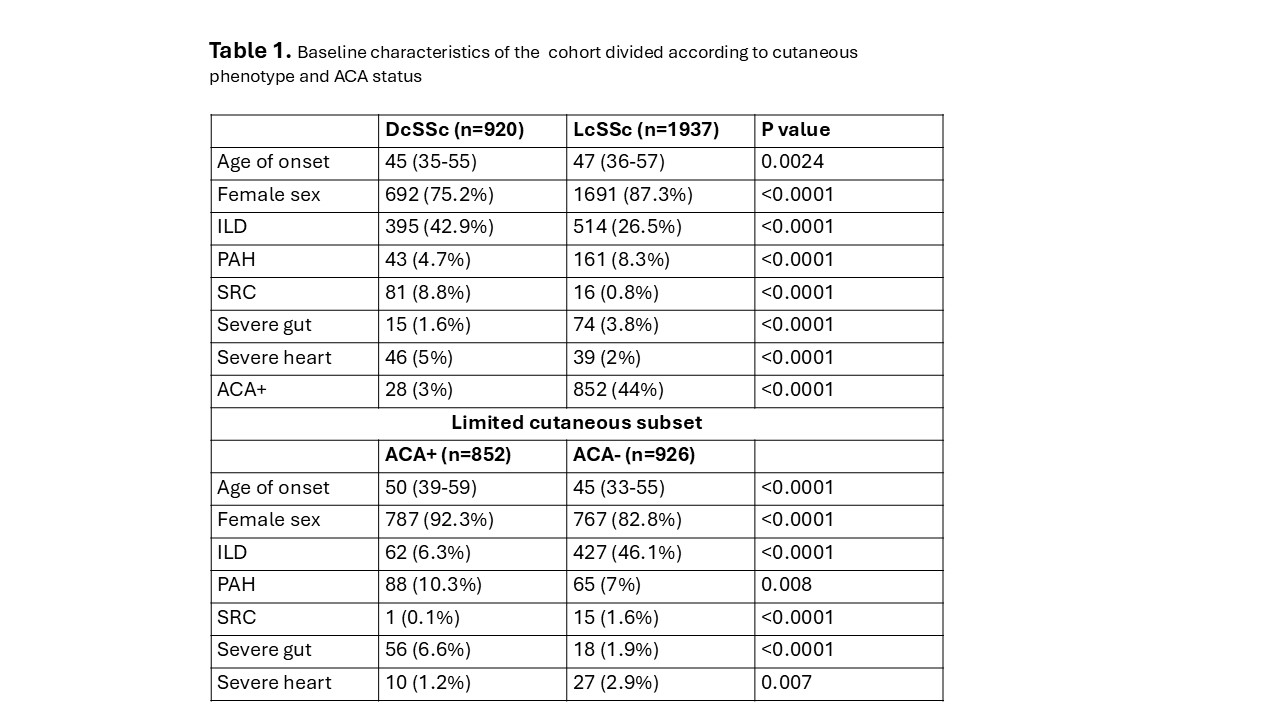
Results: Baseline characteristics of the cohort are summarised in Table 1. Around one third of cases had dcSSc and as expected this was associated with more frequent disease complications except for PAH and severe GI involvement that were most frequent in lcSSc. A total of 902 (31.6%) patients met the outcome in the first 10 years of the disease; 543 in the lcSSc group. Survival analysis revealed a significantly lower frequency of MINIMISE events in lcSSc vs DcSSc (Figure 1). Moreover, when considering only lcSSc, ACA negative patients had significantly more events over time (Figure 2).
Conclusion: In lcSSc, clinically meaningful progression, defined by the MINIMISE endpoint, is more frequent in ACA-negative patients. This subgroup may have greatest need and benefit from disease-modifying treatment, and this could be explored in a long-term event driven study although our data suggest that a large cohort and long term follow up will be essential to show benefit in lcSSc.
To cite this abstract in AMA style:
Yee P, kanitkar m, rodolfi s, Ong V, Denton C. Comparing Long-term Outcome Across Systemic Sclerosis Subgroups Using a Multi-Organ Disease Progression Score [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/comparing-long-term-outcome-across-systemic-sclerosis-subgroups-using-a-multi-organ-disease-progression-score/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparing-long-term-outcome-across-systemic-sclerosis-subgroups-using-a-multi-organ-disease-progression-score/

.jpg)
.jpg)