Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Chronic anterior uveitis (CAU) develops in 15-20% of juvenile idiopathic arthritis (JIA) cases. Early detection may prevent vision loss. However, known clinical risk factors do not accurately predict CAU. We and others report that 50% of the same protein biomarkers detected in tear fluid of patients overlapped in paired aqueous humor. Non-invasive biomarkers that reflect eye inflammation and are easily implemented in clinic may improve prediction of CAU and influence screening. We aim to compare inflammatory mediators in tear fluid and serum of children with JIA with and without CAU.
Methods: The prospective multicenter PEDIA-U study enrolled JIA children without (JIA-no-U) and with CAU (JIA-U) ≥4 y/o. Serum was paired with tear fluid collected from both eyes at same study visit using Schirmer strips. S100A8/A9 and A12 were measured by ELISA, and IL-8, CXCL-10 (IP-10), MCP-1, sICAM-1, APRIL and VEGF-A by Luminex assays in all samples. We compared levels between JIA-no-U and JIA-U per sample type, then between serum and tear fluid where we calculated tear/blood ratios to assess the difference in abundance between both samples. As samples were collected from both eyes, we used a mixed model analysis with individuals and eyes as random effects. Data was log-transformed prior to analyses.
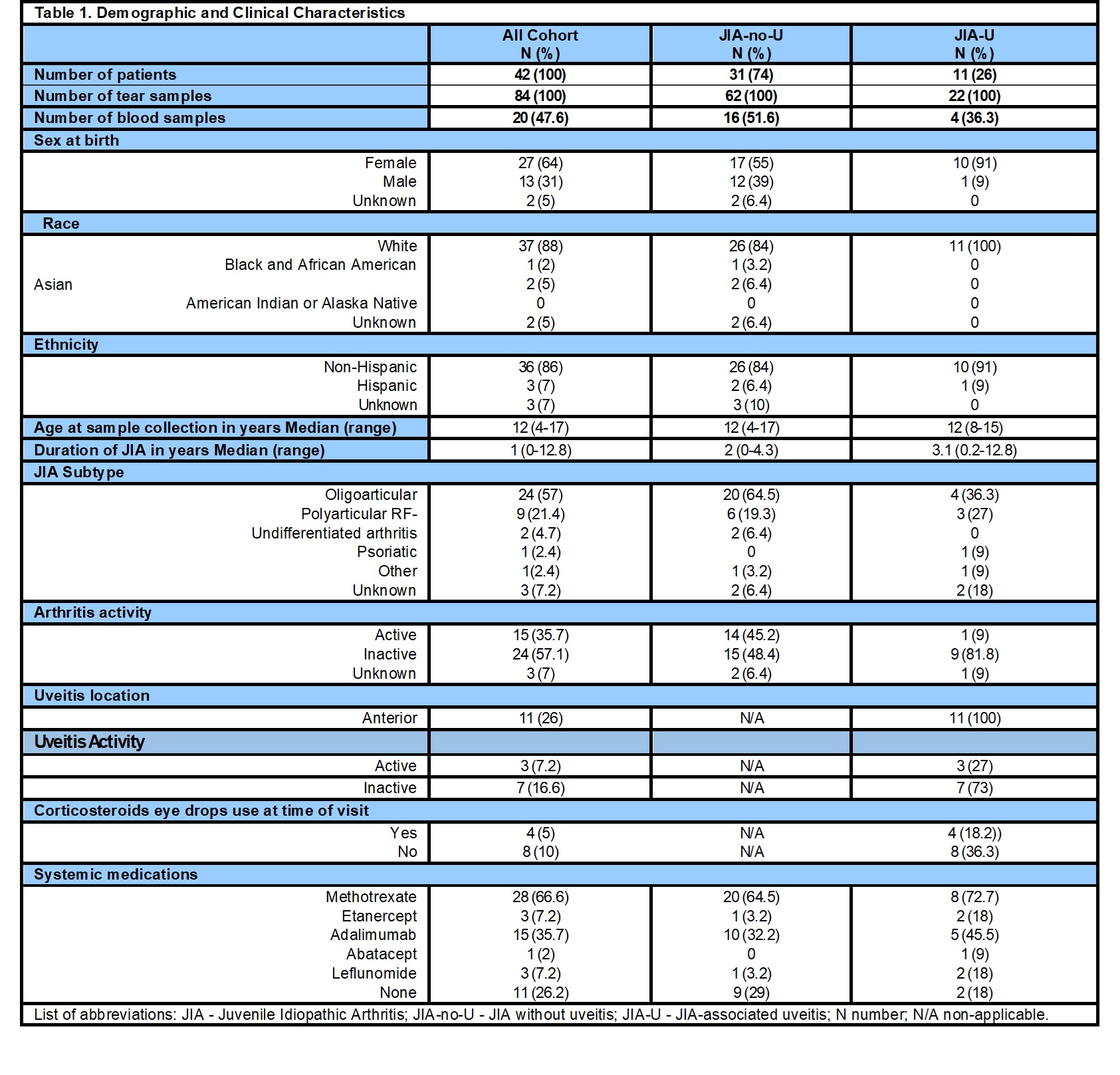
Results: Forty-two patients (31 JIA-no-U, 11 JIA-U) contributed 84 tear (62 JIA-no-U, 22 JIA-U) and 20 serum samples (16 JIA-no-U, 4 JIA-U) (Table 1). Two JIA-no-U patients at time of collection later developed CAU but were grouped as JIA-U. In serum, S100A12 (JIA-no-U LS mean 76,807.11 vs JIA-U LS mean 27,455.02, p=0.0027) and S100A8/A9 (JIA-no-U LS mean 5,608.29 vs JIA-U LS mean 2,217.52, p=0.0008) were elevated in JIA-no-U (Table 2). In tears, CXCL-10 (JIA-no-U LS means 5.1 vs JIA-U LS means 4.01, p=0.001) and MCP1 (JIA-no-U LS Means 2.18 vs JIA-U LS means 1.31, p= 0.0083) were elevated in JIA-no-U (Table 2). Comparing paired serum and tears levels in 19 patients (Table 3), S100A12 (serum mean 4.67 vs tears mean 3.09, ratio 0.66 p< 0.001) and sICAM-1 (4.24 vs 3.55, 0.83 p< 0.001) were significantly elevated in serum. Conversely, CXCL10 (serum 0.09 vs tear 2.14, ratio 22.76 p< 0.001), APRIL (1.88 vs 3.55, 1.86 p< 0.001), and VEGF (2.2 vs 2.69, 1.22 p< 0.003) were elevated in tear fluid. MCP-1 and S100A8/9 were similar in both samples (21.06 vs 0.86, 0.811 p=0.292 and 3.54 vs 3.66, 0.393 p=0.393 respectively).
Conclusion: We compared levels of tear and serum-based biomarkers in children with JIA with and without CAU. Differences in S100 proteins in serum may reflect systemic inflammation. CXCL10, APRIL and VEGF were highly enriched in tear fluid, while MCP-1 and S100 proteins were similar in both samples. These tear-based biomarkers may reflect damage from inflammation, allowing an influx of inflammatory mediators into the eye. Importantly, biomarkers reported in JIA and JIA-U were detected in both sample types. Elevated baseline S100A12 in JIA children who develop CAU has been noted, as well as VEGF in cystoid macular edema. Tear and blood-based biomarkers are easily measured in various clinical settings, may complement known CAU risk factors, and improve screening. Our future studies will consider disease activity and topical and systemic treatment.
.jpg) Table 2: Cytokine and chemokines in serum and tears of children with JIA-U and JIA-no-U, Based on natural logarithm transferred analysis, using Wilcoxon Two Sample Exact Test. In bold the significant cytokines and chemokines
Table 2: Cytokine and chemokines in serum and tears of children with JIA-U and JIA-no-U, Based on natural logarithm transferred analysis, using Wilcoxon Two Sample Exact Test. In bold the significant cytokines and chemokines
.jpg) Table 3: Comparison between tears and blood level of logarithmic value of biomarkers
Table 3: Comparison between tears and blood level of logarithmic value of biomarkers
To cite this abstract in AMA style:
Maccora I, Pavlenko M, Altaye M, Brunner H, Chang M, Cooper A, Davidson S, Duell A, Gangwani B, Hersh A, Holland G, Langefeld C, Lerman M, Lo M, Miraldi Utz V, Prahalad S, Schulert G, Quinlan-Waters M, Stahl E, Tsui E, Angeles-Han S. Comparing biomarkers associated with uveitis in tear fluid and serum samples of children with Juvenile Idiopathic Arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/comparing-biomarkers-associated-with-uveitis-in-tear-fluid-and-serum-samples-of-children-with-juvenile-idiopathic-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparing-biomarkers-associated-with-uveitis-in-tear-fluid-and-serum-samples-of-children-with-juvenile-idiopathic-arthritis/