Session Information
Date: Saturday, November 16, 2024
Title: Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Previous work has shown unique gene expression profiles in muscle tissue corresponding to the anti-synthetase syndrome, with an emphasis on interferon gene signatures (Type I and Type II) and other genes (e.g., SAA1, SPP1, CXCL9) that reflect the predominant role of macrophages and T cells in this myositis subtype. We therefore assessed transcriptional profiles in our established murine model of Histidyl-tRNA synthetase (HRS)-induced myositis to determine the degree of pathway overlap with human disease.
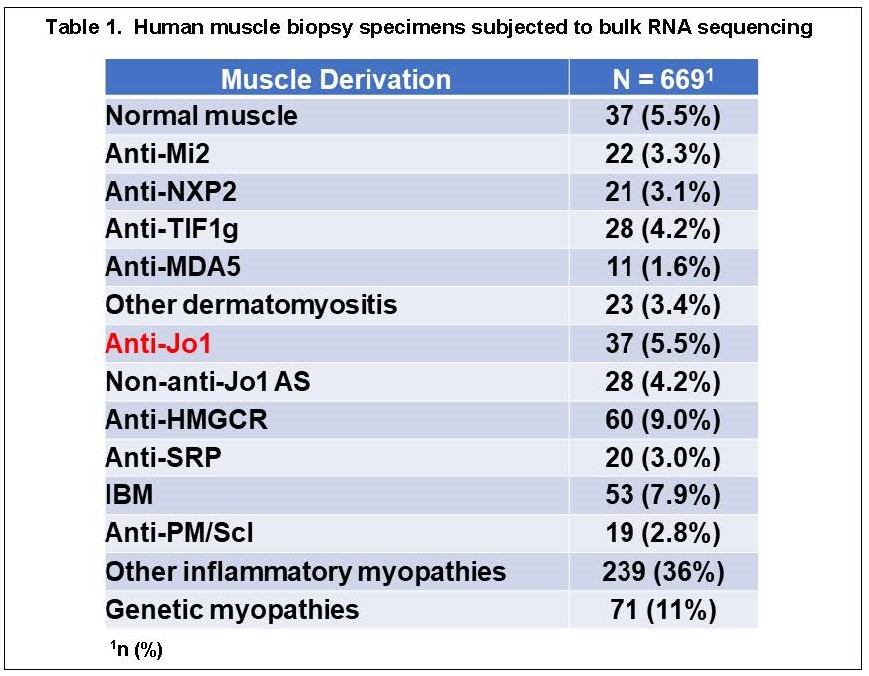
Methods: Bulk RNA sequencing was performed in 669 human muscle biopsy specimens corresponding to different clinical subsets of idiopathic inflammatory myopathy as well as healthy controls and genetic myopathies (Table 1). In parallel, bulk RNA sequencing was performed on muscle tissue harvested from mice 14 days post immunization with recombinant HRS versus PBS. Profiles of differentially expressed genes (DEGs) were generated by comparing genes expressed in human Jo-1 muscle tissue to those expressed in other myositis subsets and healthy controls. The degree of overlap with genes induced in mouse muscle tissue following immunization with recombinant HRS was quantified via summary statistics that included Chi-square testing with Yates correction. Gene Ontology algorithms were used to assess pathways corresponding to shared DEGs between muscle tissue derived from humans with Jo-1+ anti-synthetase syndrome and mice with HRS-induced myositis.
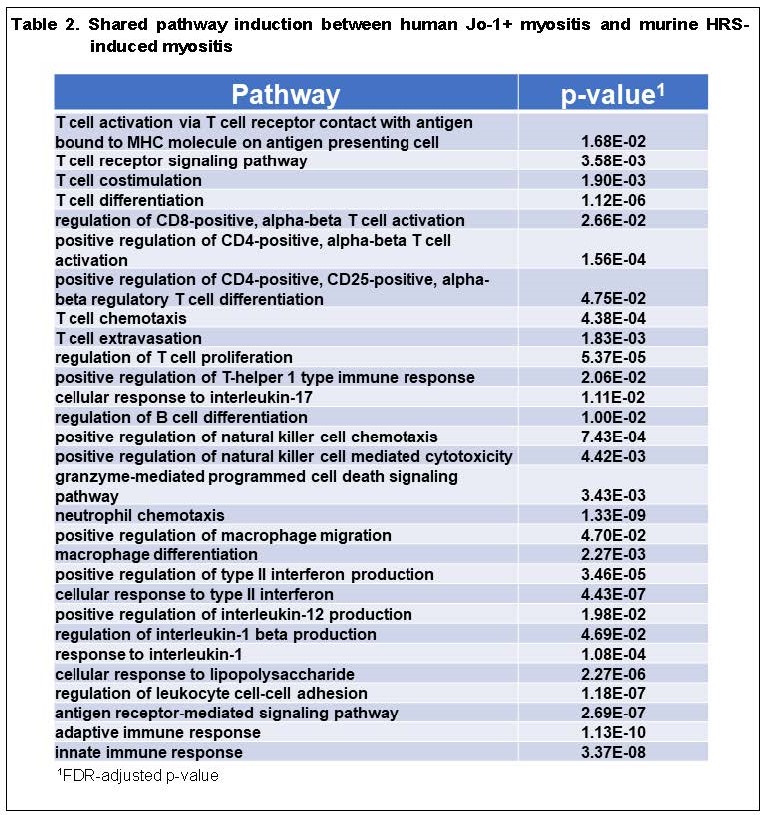
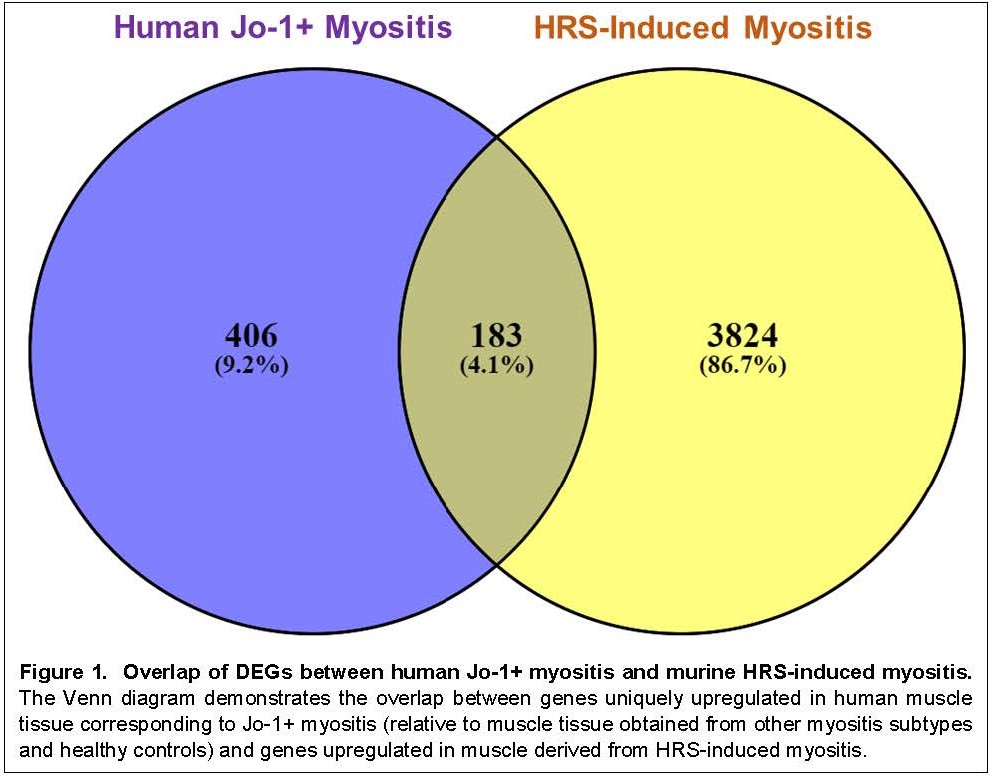
Results: 12.6% (998/7939) of the genes that were differentially expressed in murine muscle tissue following HRS immunization were also differentially expressed in human muscle tissue derived from Jo-1+ anti-synthetase syndrome. Conversely, 7.4% (454/6159) of the genes that were not differentially expressed in the muscle tissue from mice immunized with HRS were differentially expressed in muscle tissue corresponding to Jo-1+ anti-synthetase syndrome (p-value < 2.2e-16). More detailed comparison of DEGs in human Jo-1+ anti-synthetase syndrome and HRS-induced myositis (using a log2 fold change cutoff of 1) yielded 183 genes that were upregulated in both human and murine muscle tissue, representing 31% of the genes preferentially expressed in human Jo-1+ anti-synthetase syndrome (Figure 1). Gene Ontology overrepresentation analysis indicated that the 183 shared DEGs contributed to a number of non-immune pathways involved in DNA replication and cell cycle progression as well as immune pathways corresponding to Type II interferon signaling, macrophage and NK cell activation, peptide processing/antigen presentation, TCR signaling, and TH1 differentiation/proliferation (Table 2).
Conclusion: Comparison of transcriptional profiles between human muscle tissue derived from different myositis subsets and murine muscle tissue immunized with HRS reveals significant overlap with human Jo-1+ myositis specimens, validating HRS-induced myositis as a model of the anti-synthetase syndrome and highlighting the role of Type II interferon signaling, macrophage activation/chemotaxis, and TH1-predominant T cell responses. Collectively, these data support key roles for both innate and adaptive immune activation in the anti-synthetase syndrome.
To cite this abstract in AMA style:
Pinal-Fernandez I, Reay D, Oriss T, Pak k, Casal-Dominguez M, milisenda j, Selva-O’Callaghan A, Stenzel W, Mammen A, Ascherman D. Comparative Transcriptional Profiling Reveals Shared Pathway Activation Between Human Jo-1+ Anti-Synthetase Syndrome and Murine Histidyl-tRNA Synthetase-Induced Myositis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/comparative-transcriptional-profiling-reveals-shared-pathway-activation-between-human-jo-1-anti-synthetase-syndrome-and-murine-histidyl-trna-synthetase-induced-myositis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-transcriptional-profiling-reveals-shared-pathway-activation-between-human-jo-1-anti-synthetase-syndrome-and-murine-histidyl-trna-synthetase-induced-myositis/