Background/Purpose: Rheumatology societies recommend serum-urate (SU)-driven treat-to-target (TTT) strategies for the management of gout. However, cardiovascular (CV) safety of urate-lowering therapy (ULT) has been questioned. It is not fully understood whether SU-driven intensive TTT strategies are safe compared to less intensive usual care. We sought to examine the comparative safety of intensive TTT vs. less intensive strategies using observational real-world and causal inference techniques.
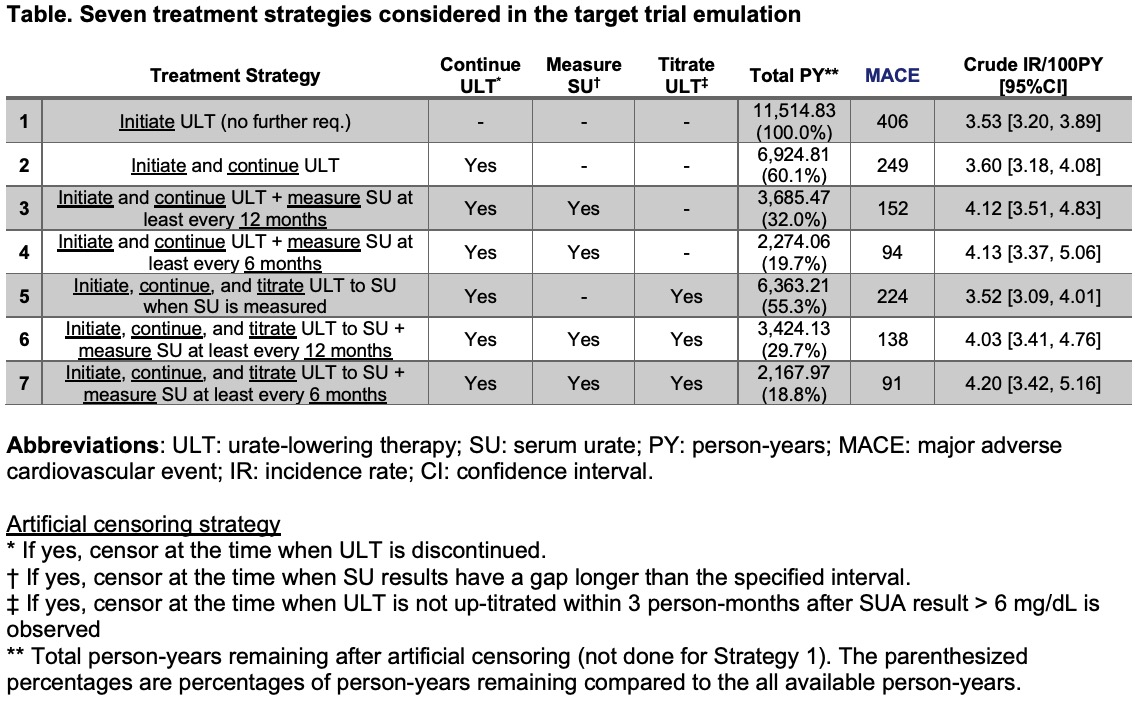
Methods: Using Medicare claims data (2007–2016) linked to EHR from 2 health care provider networks, we identified patients newly diagnosed with gout who initiated allopurinol or febuxostat. We emulated a hypothetical target trial that compared the risk of major adverse CV events (i.e., myocardial infarction, stroke, or cardiovascular mortality) of gout patients receiving 7 different TTT strategies (Table). Three aspects of TTT were considered in identifying different treatment strategies that we compared: (1) continuation of ULT, (2) regular SU monitoring, and (3) timely modification of ULT if SU >6 mg/dL. We applied “cloning” methods to avoid immortal time bias and performed inverse probability (IP) weighted analysis to account for both baseline and time-varying confounding.
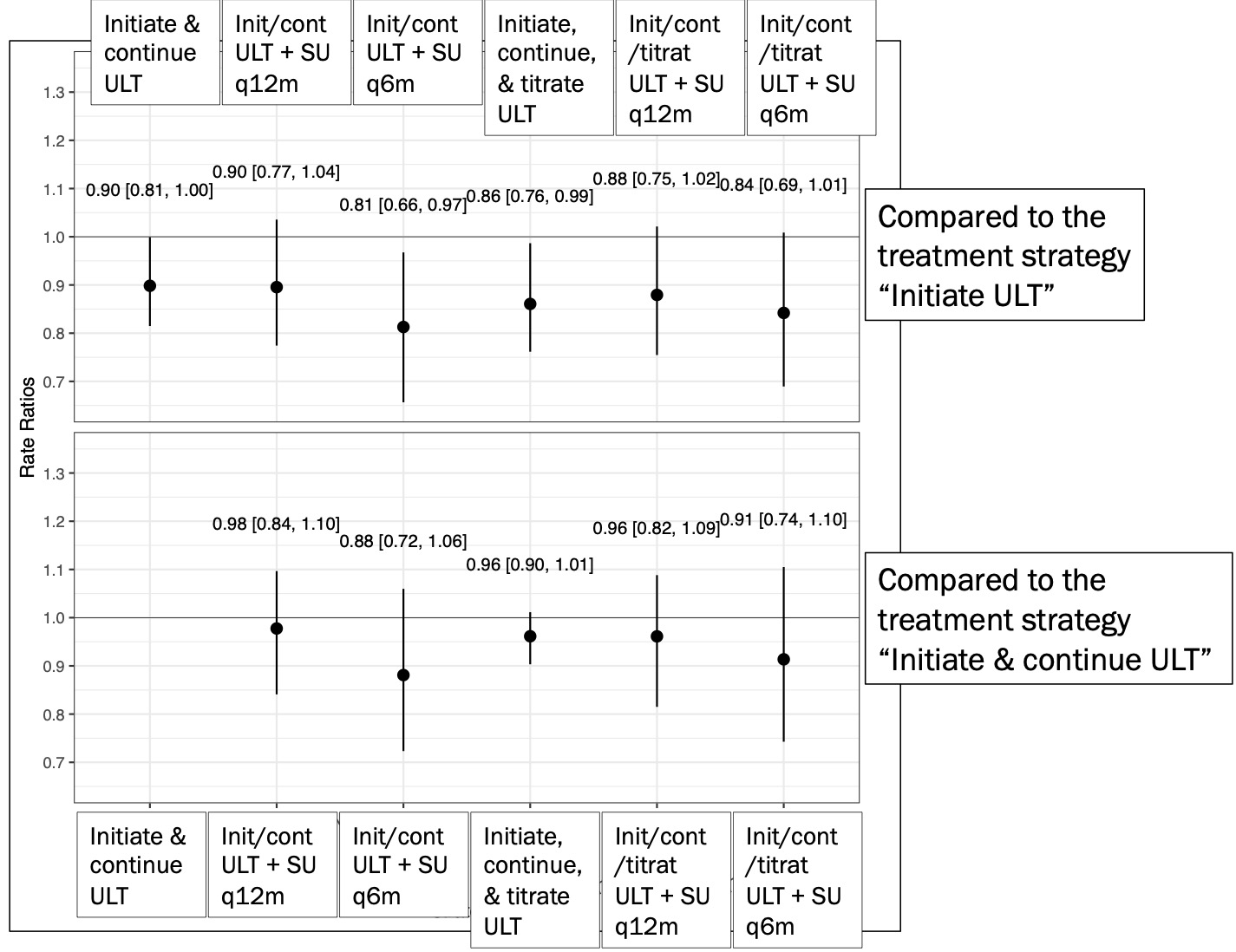
Results: We identified a total of 4,402 patients who had a diagnosis of gout and initiated either allopurinol or febuxostat (mean age 76.9; male 60%). During 11514.83 person-years (PY) under ‘Initiate ULT’ strategy (Table), the incidence rate (IR) of MACE was >3.53 per 100 PY (95%CI, 3.20-3.89). Compared to this “Initiate ULT” strategy (no specification beyond ULT initiation) (Figure- upper panel), the “Initiate and continue ULT + SU monitoring every 6 months” strategy was associated with decreased risk of MACE (RR 0.81, 95%CI 0.66-0.97]. Similarly, “Initiate, continue, and titrate ULT to SU” strategy had a RR of 0.86 (95%CI 0.76, 0.99]. When comparison was made to the “Initiate and continue ULT” strategy (Figure- lower panel), the risk of MACE was similar across 5 other strategies.
Conclusion: In this large cohort of older patients with gout who newly started ULT, we found a decreased MACE rate with the TTT strategy for gout compared to only initiating ULT regardless of medication adherence. However, similar MACE risk was noted across different TTT strategies for gout compared to those who initiated and continued ULT. Despite rigorous causal inference methods that we used, future randomized clinical trials are needed to confirm our results.
Abbreviations: ULT: urate-lowering therapy; SU: serum urate; PY: person-years; MACE: major adverse cardiovascular event; IR: incidence rate; CI: confidence interval.
Artificial censoring strategy
* If yes, censor at the time when ULT is discontinued.
† If yes, censor at the time when SU results have a gap longer than the specified interval.
‡ If yes, censor at the time when ULT is not up-titrated within 3 person-months after SUA result > 6 mg/dL is observed
** Total person-years remaining after artificial censoring (not done for Strategy 1). The parenthesized percentages are percentages of person-years remaining compared to the all available person-years.
To cite this abstract in AMA style:
Yoshida K, Liu J, Solomon D, Glynn R, Kim S. Comparative Safety of Gout “Treat-to-target” and “Usual Care” Treatment Strategies on Cardiovascular Outcomes Using Observational Data: Causal Inference Approach [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/comparative-safety-of-gout-treat-to-target-and-usual-care-treatment-strategies-on-cardiovascular-outcomes-using-observational-data-causal-inference-approach/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-safety-of-gout-treat-to-target-and-usual-care-treatment-strategies-on-cardiovascular-outcomes-using-observational-data-causal-inference-approach/