Session Information
Session Type: Abstract Session
Session Time: 5:00PM-5:50PM
Background/Purpose: Immunometabolism is now recognaized to be crucial in the pathogenesis of rheumatoid arthritis (RA). We have recently shown that the expression of glutaminase 1 (GLS1), a key enzyme in glutaminolysis, is upregulated in fibroblast-like synoviocytes from RA patients (RA-FLS) and that GLS1 inhibition suppresses RA-FLS proliferation (1). However, glutaminolysis has been known to suppress autophagy by activating mTORC1 or counteracting ROS production (2). Given the possibility of autophagy upregulation following glutamiolysis inhibition, therapies targeting both autophagy and glutaminolysis may be more effective in suppressing cell growth of RA-FLS, yet the relation between glutaminolysis and autophagy in RA-FLS has not been investigated. Here we examined the effects of inhibiting both glutaminolysis and autophagy on RA-FLS and autoimmune arthritis in SKG mice.
Methods: GLS1 inhibitor, compound 968 (C968), was used to suppress glutaminolysis, and Chloroquine (CQ) was used to inhibit autophagy. To detect autophagy, the expression of ATG5 and LC3B was measured by real-time PCR and the production of LC3-Ⅱ was analyzed by Western blotting. The formation of autophagic vacuoles was identified by immunfluorescense. Cell growth was evaluated by BrdU assay. Apoptosis was analyzed by flow cytometry staining with Annexin V-FITC and PI. C968 and CQ were administered subcutaneously to Zymosan A-injected SKG mice.
Results: C968 upregulated the expression of ATG5 and LC3B, and increased the protein level of LC3-Ⅱ in RA-FLS. C968 also facilitated autophagosome formation. These results suggested that inhibition of glutaminolysis promoted autophagy in RA-FLS. The combined treatment with C968 and CQ significantly suppressed cell proliferation of RA-FLS more strongly than did C968 or CQ alone. In addition, C968 combined with CQ increased the apoptosis rate, whereas either C968 or CQ alone did not. Furthermore, combination of C968 and CQ significantly attenuated the degree of arthritis in SKG mice, while C968 or CQ monotherapy did not (Figure).
Conclusion: The GLS1 inhibitor C968 promotes autophagy in RA-FLS. C968 in combination with CQ reduces proliferation and enhances apoptosis in RA-FLS, and ameliorates the arthritis in SKG mice. Suppressing C968-induced autophagy may be a promising therapy for arthritis.
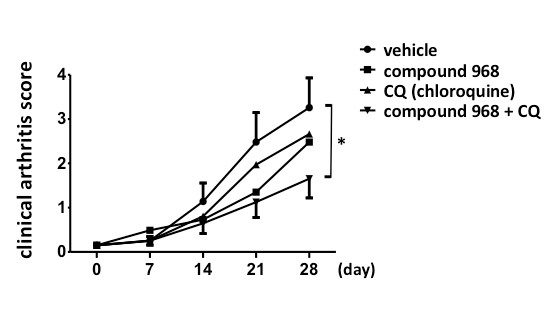 Combination of C968 and CQ significantly attenuated the degree of arthritis in SKG mice.
Combination of C968 and CQ significantly attenuated the degree of arthritis in SKG mice.
To cite this abstract in AMA style:
Naka I, Saegusa J, Uto K, Yamamoto Y, Ichise Y, Yamada H, Ueda Y, Okano T, Takahashi S, Sendo S, Morinobu A. Combined Inhibition of Autophagy and Glutamine Metabolism Suppresses Cell Growth of RA Synoviocytes and Ameliorates Arthritis in SKG Mice [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/combined-inhibition-of-autophagy-and-glutamine-metabolism-suppresses-cell-growth-of-ra-synoviocytes-and-ameliorates-arthritis-in-skg-mice/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/combined-inhibition-of-autophagy-and-glutamine-metabolism-suppresses-cell-growth-of-ra-synoviocytes-and-ameliorates-arthritis-in-skg-mice/
