Session Information
Date: Monday, November 13, 2023
Title: (1221–1255) Pediatric Rheumatology – Clinical Poster II: Connective Tissue Disease
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
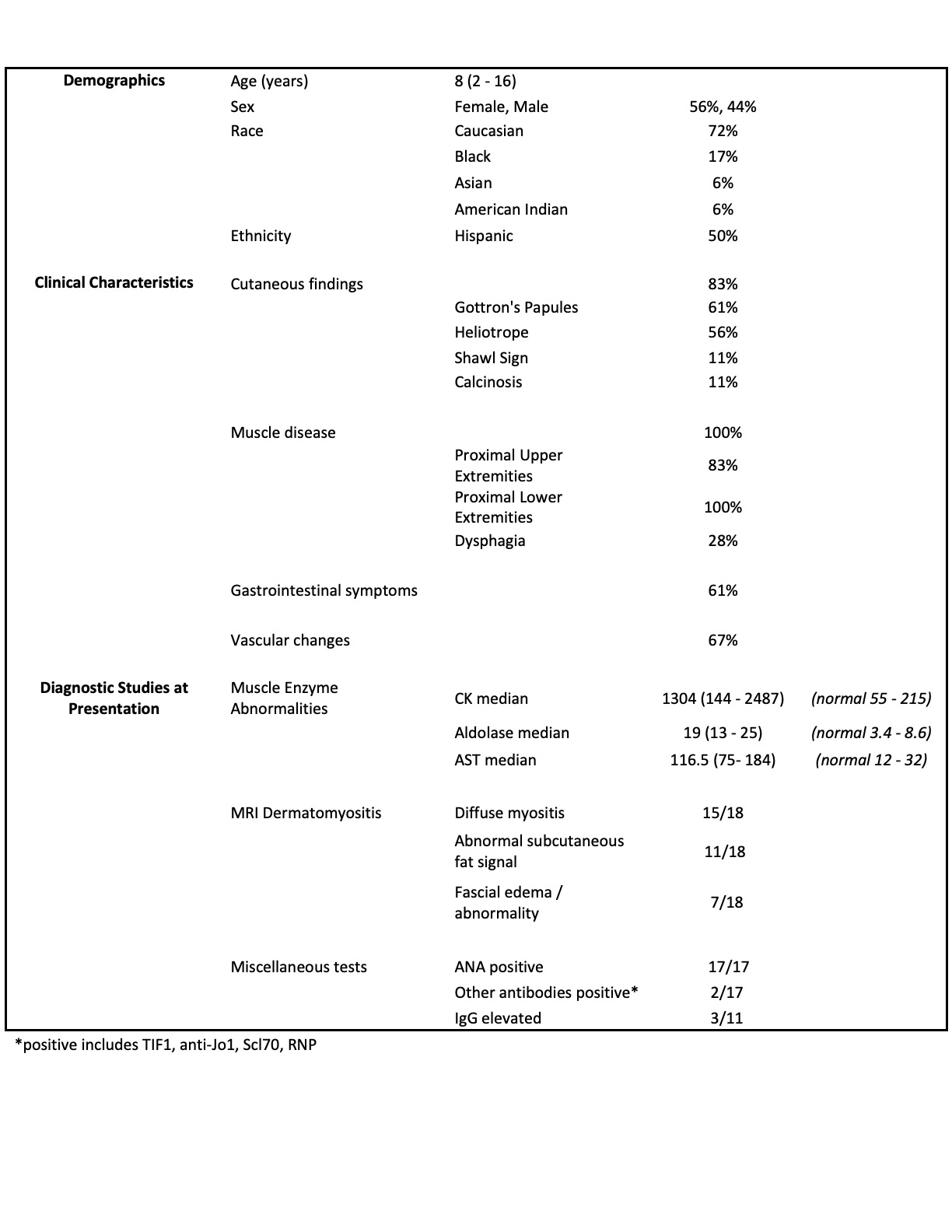
Background/Purpose: Juvenile dermatomyositis (JDM) is a rare idiopathic inflammatory myopathy with clinically heterogeneous presentations that can be categorized by myositis-specific antibodies (MSAs). NXP2 is among the more common of MSAs, seen in approximately 20-25% of cases. It is associated with an increased risk of severe muscle weakness, dysphagia, and vasculopathy which may cause gastrointestinal ulceration and bleeding. Children with NXP2 are described to be more resistant to conventional therapies and at risk for prolonged active disease. This retrospective chart review aims to provide additional data on 18 patients at one institution regarding clinical presentation, management, and outcomes.
Methods: With approval from our IRB, we reviewed the presentation, management, and outcomes of patients with positive NXP2-antibody JDM before 18 years old between January 2012 – May 2023. Data abstracted include clinical features, diagnostic studies, therapy at initial and follow up at 6-month, 12-month, and last clinic visit. Muscle strength was measured by muscle manual testing (MMT) and Childhood Myositis Assessment Scale (CMAS). Remission defined as CMAS >48 and/or documented full strength, absence of active skin and vascular disease, and normalized muscle enzymes.
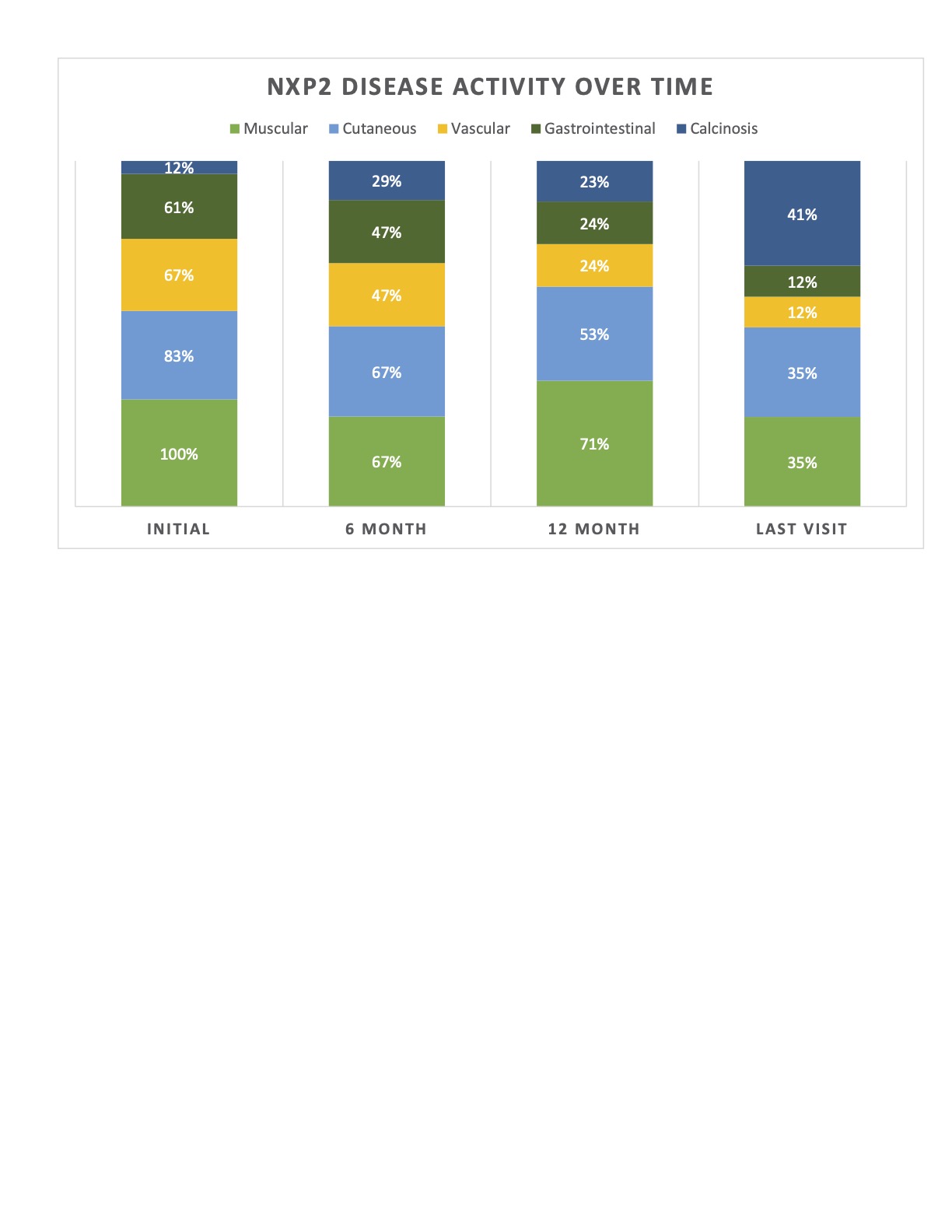
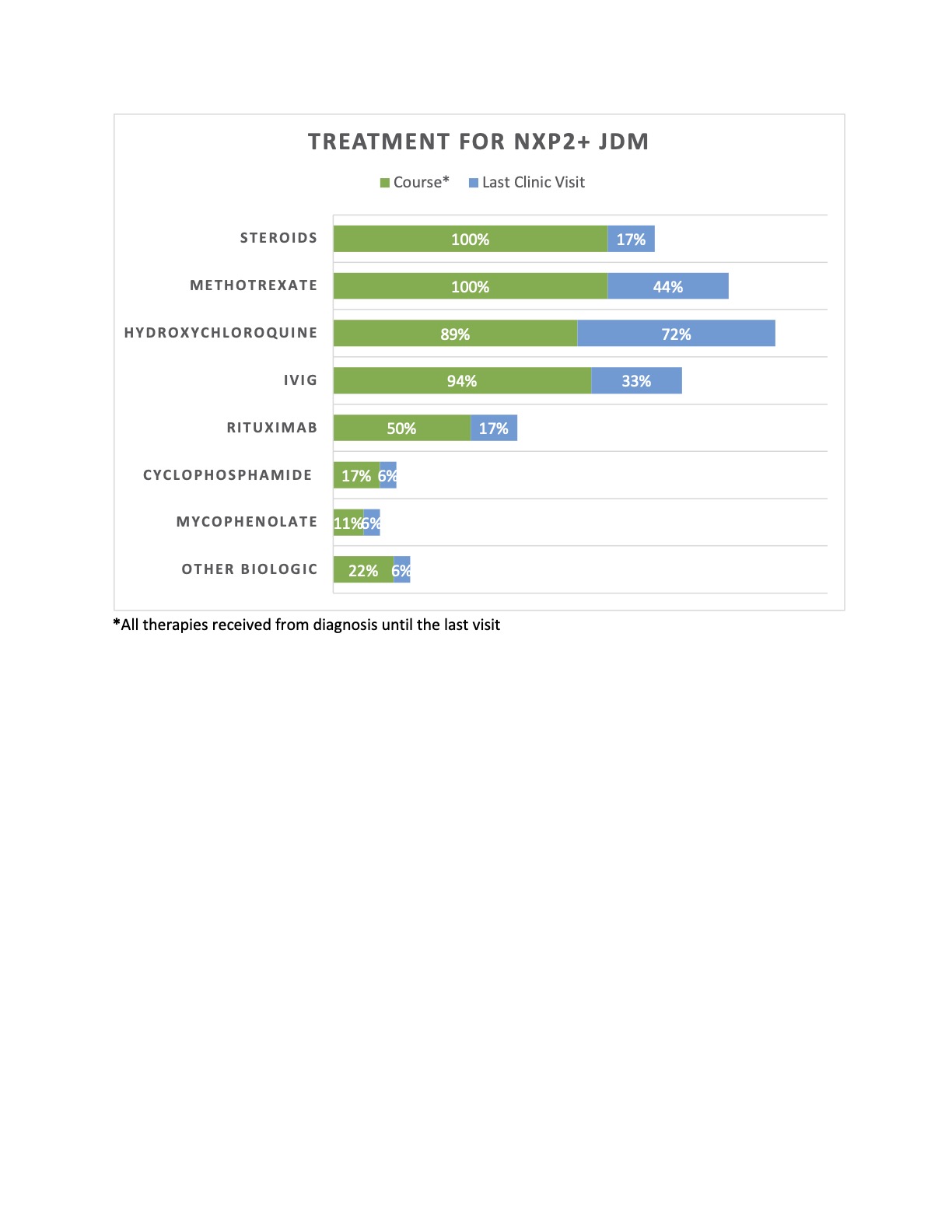
Results: A total of 18 patients were included: 66% female, 50% Hispanic, with a median age of 8 years [4-12] at presentation; median time to diagnosis was 1.5 months [1-3]. All had progressive proximal lower extremity and neck muscle weakness. Proximal upper extremity weakness was seen in 83%, with bulbar weakness in 44% (see Figure 1). Initial median CMAS was 12 [10-18]. All had abnormal MRIs, with 83% showing diffuse myositis. 56% of patients had a muscle biopsy, which all but 1 were diagnostic of JDM. 83% of patients presented with cutaneous manifestations, two with calcinosis. 61% had periungual vascular changes. Gastrointestinal features were seen in 61%. Median duration of follow up was 36.5 months [24 –77]. CMAS at 12 months and the last visit were 43 and 48 [43-52], respectively. 56% reported dysphagia over time. Two patients required invasive feeding methods. Two developed skin ulcerations, 1 had bowel ulceration with perforations requiring partial colectomy with end-ileostomy creation, and 2 developed SMA syndrome from excessive weight loss. No lung or cardiac disease reported. Only 2 patients had a monophasic course. Progressive calcinosis was seen in 41% (see Figure 2). All received steroids and methotrexate, 94% IVIG, 89% hydroxychloroquine, 50% rituximab, and 22% required other biologic therapies (see Figure 3). Remission achieved in 44% after a median of 44 months of therapy [25.5 – 52.5]. No malignancy or deaths reported.
Conclusion: Children with NXP2+ JDM in our cohort developed a chronic disease course where gastrointestinal features were common with complications that could be severe. Progressive calcinosis was seen despite aggressive therapies. Remission was achieved in 44% after prolonged therapy. Our patients provided information as to the challenges for supportive and multimodal immunotherapy. Additional large multi-center cohort studies are needed to determine the optimum treatment course with validated remission criteria.
To cite this abstract in AMA style:
Molina S, Gist D, De Guzman M, Muscal E, Lai J, Pereira M. Clinical Characteristics and Disease Outcomes of anti-NXP2 Positive Juvenile Dermatomyositis: A Single Center Cohort [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/clinical-characteristics-and-disease-outcomes-of-anti-nxp2-positive-juvenile-dermatomyositis-a-single-center-cohort/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-characteristics-and-disease-outcomes-of-anti-nxp2-positive-juvenile-dermatomyositis-a-single-center-cohort/