Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Primary antiphospholipid syndrome (PAPS) is an autoimmune disorder characterized by the presence of pathogenic autoantibodies. The key immune cell subsets change in PAPS patients remains unclear.
Methods: We enrolled 35 primary APS (PAPS) patients fulfilling the 2006 Sydney criteria, five antiphospholipid antibody (aPLs) carriers, and 10 healthy controls (age-, gender-matched). Peripheral blood mononuclear cells (PBMCs) were analyzed using Mass Cytometry (CyTOF) with 42 markers to identify immune cell subsets. Plasma cytokine profiling of 55 PAPS patients was performed using the procartaPlex 65 panel assay. Peripheral B cells from PAPS patients and healthy individuals were isolated for RNA sequencing (RNAseq). Flow cytometry and RT-qPCR were used to validation. FlowJo and R software packages were utilized for CyTOF data analysis.
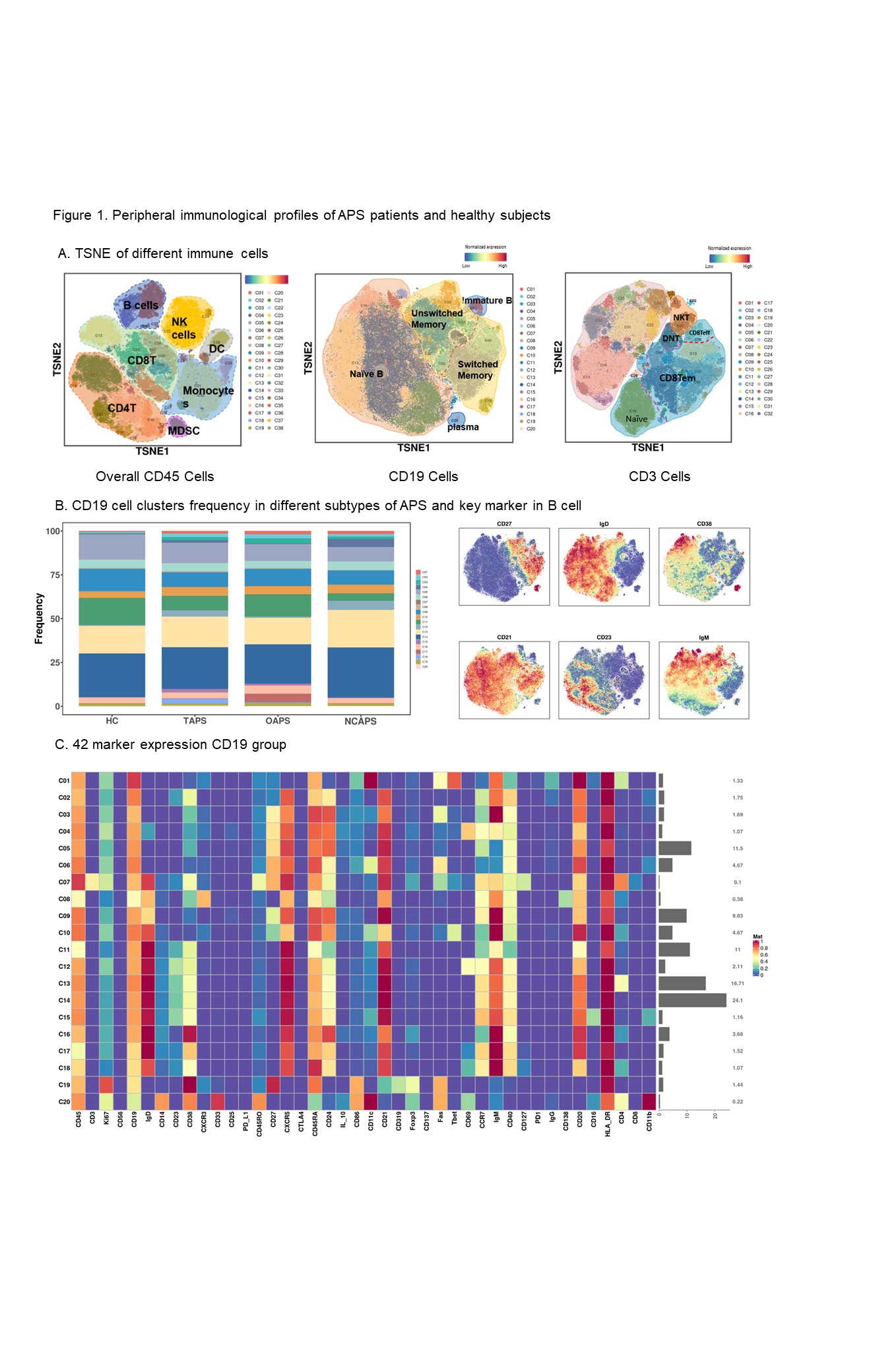
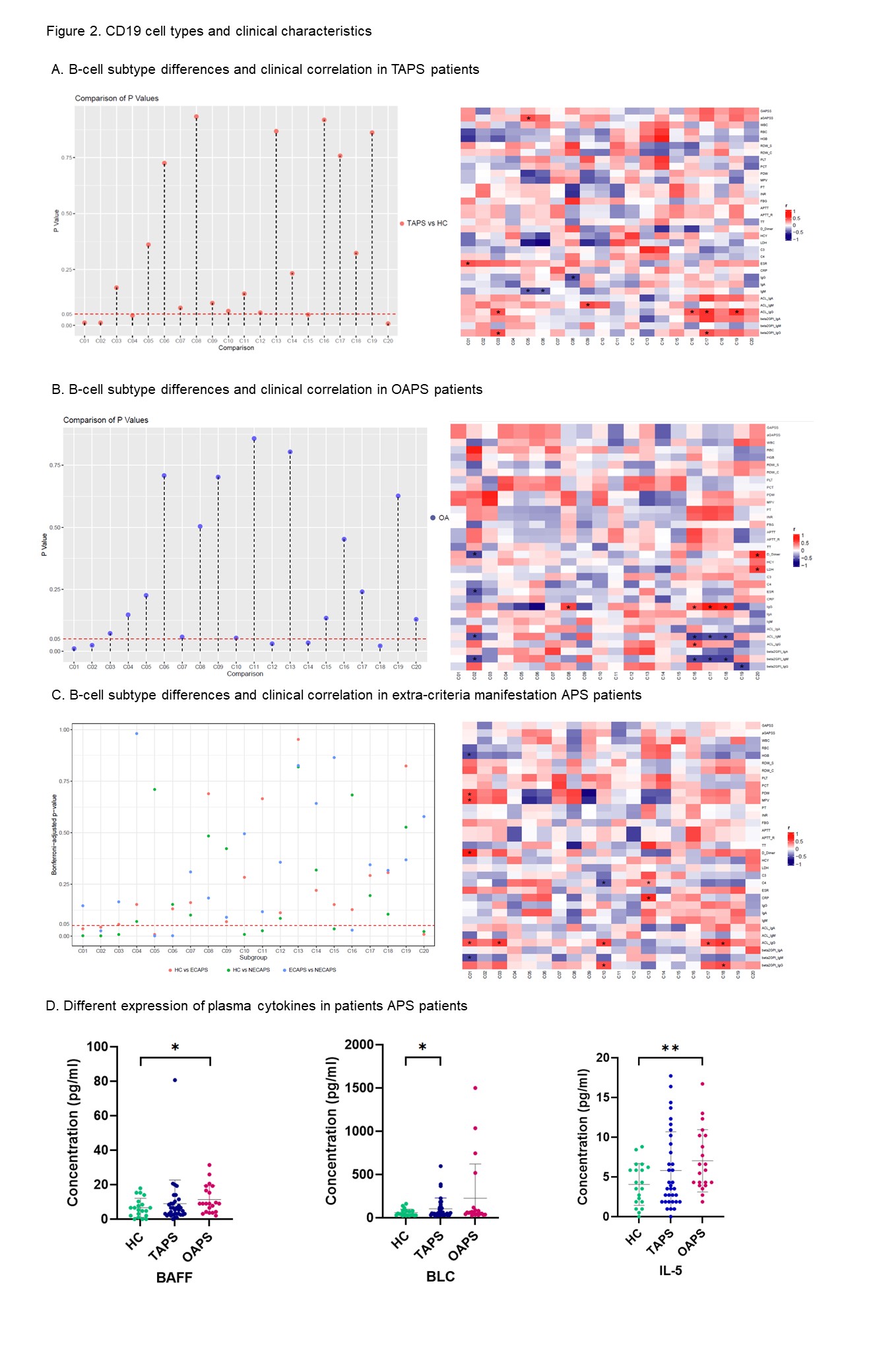
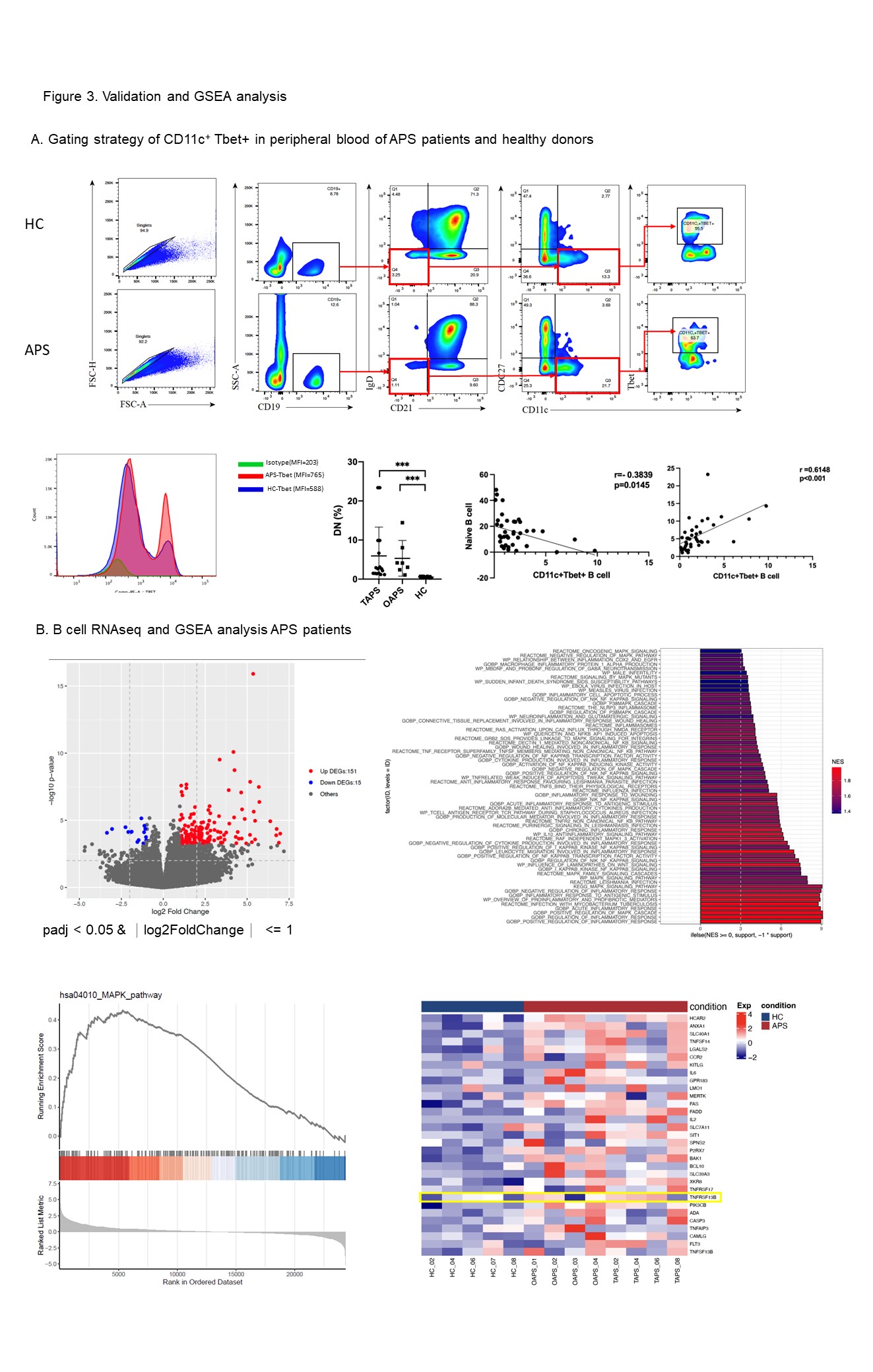
Results: Our study investigated that APS patients had a significant difference in peripheral blood subtype distribution . Using CD19 as a baseline for dimensionality reduction clustering, we identified 20 subgroups of B cells (Figure 1). Naïve B cells, characterized by IgD+CD27-CD38+CD24+, were the predominant B cell subset in APS patients (C14, 21.04%), while plasmablasts accounted for 1.55%. C01 and C02 subpopulations within B cells were significantly increased in the peripheral circulation of patients (C01: 1.89±1.68 vs 0.58±0.41, P=0.02; C02: 2.16±1.78 vs 0.71±0.67, P=0.015; Figure 2). Both were characterized as IgD-CD27- double negative B cells, with C01 expressing high levels of CD11c and Tbet, which are a subset of age-associated B cells (ABC). In APS patients, C01 cells showed a positive correlation with ESR. C02 showed a positive association with β2GPI IgG expression in patients without extra-criteria organ involvement. We further validated these findings by expanding the sample size and analyzing an additional 23 APS patients using flow cytometry (Figure 3A). The results demonstrated a significant increase in DN B cells in APS patients, along with a marked increase in the CD11cTbet signaling intensity of DN B cells (Figure 2). This suggests that DN cells, especially ABC cells, may be involved in the sustained production of high levels of aPLs in APS patients. Interestingly, we found that the C19 (IgD-CD27++CD38+++CD24-) plasmablasts showed a negative correlation with the production of IgG subtype antibodies in OAPS patients, which contrasts with TAPS patients. This suggests that different mechanisms may be involved in B cell participation in APS with different phenotypes. Based on the results of plasma cytokine analysis, we observed elevated expression of BLC/CXCL13 and BAFF in the plasma of APS patients. We then identified upregulation of TNFRSF13B, a gene associated with the Transmembrane activator and CAML interactor (TACI) protein by RNAseq. Furthermore, GSEA analysis revealed activation of the MAPK and NF-κB pathways within B cells (Figure 3B).
Conclusion: Double-negative B cells are significantly higher in APS patients, where ABC cells may play a role in aPLs production. The aberrant differentiation and maturation of B cells in APS patients may be triggered by TACI downstream cascade.
To cite this abstract in AMA style:
Long Y, zhao J, Li M, Zeng X. Characterization of B-Cell Subsets in Antiphospholipid Syndrome Patients: Implications for Disease Phenotype and Pathogenesis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/characterization-of-b-cell-subsets-in-antiphospholipid-syndrome-patients-implications-for-disease-phenotype-and-pathogenesis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/characterization-of-b-cell-subsets-in-antiphospholipid-syndrome-patients-implications-for-disease-phenotype-and-pathogenesis/