Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Peresolimab is a humanized immunoglobulin G1 monoclonal antibody that stimulates human programmed cell death protein 1 (PD-1). Peresolimab demonstrated superiority to placebo in Disease Activity Score-28 with CRP (DAS28-CRP) improvements from baseline at Week 12 in a Phase 2a placebo-controlled, double-blind, randomized clinical trial (NCT04634253) in adult patients with moderate to severe Rheumatoid Arthritis (RA).1 We report here, changes in exploratory biomarkers from this study.
Methods: Ninety-eight adult patients with moderate-to-severe RA (defined per American College of Rheumatology [ACR] criteria) were randomized 2:1:1 to peresolimab 700mg, 300mg, or placebo for 12 weeks. Whole blood, serum, and plasma were collected at baseline through Week 14 (end of the placebo control period). Cells were stained for CD3, CD4, CD8, CD19, CD16/56, and PD-1, processed and analyzed using standard flow cytometry methods. Serum samples were analyzed using the Olink Explore3072 panel which includes proteins that were detected in at least 25% of patients. Thirty age- and sex-matched healthy control samples were included for comparison. A gene set enrichment analysis approach was used to analyze proteomic results, and analyses were performed on comparing the combined peresolimab dose groups versus placebo.
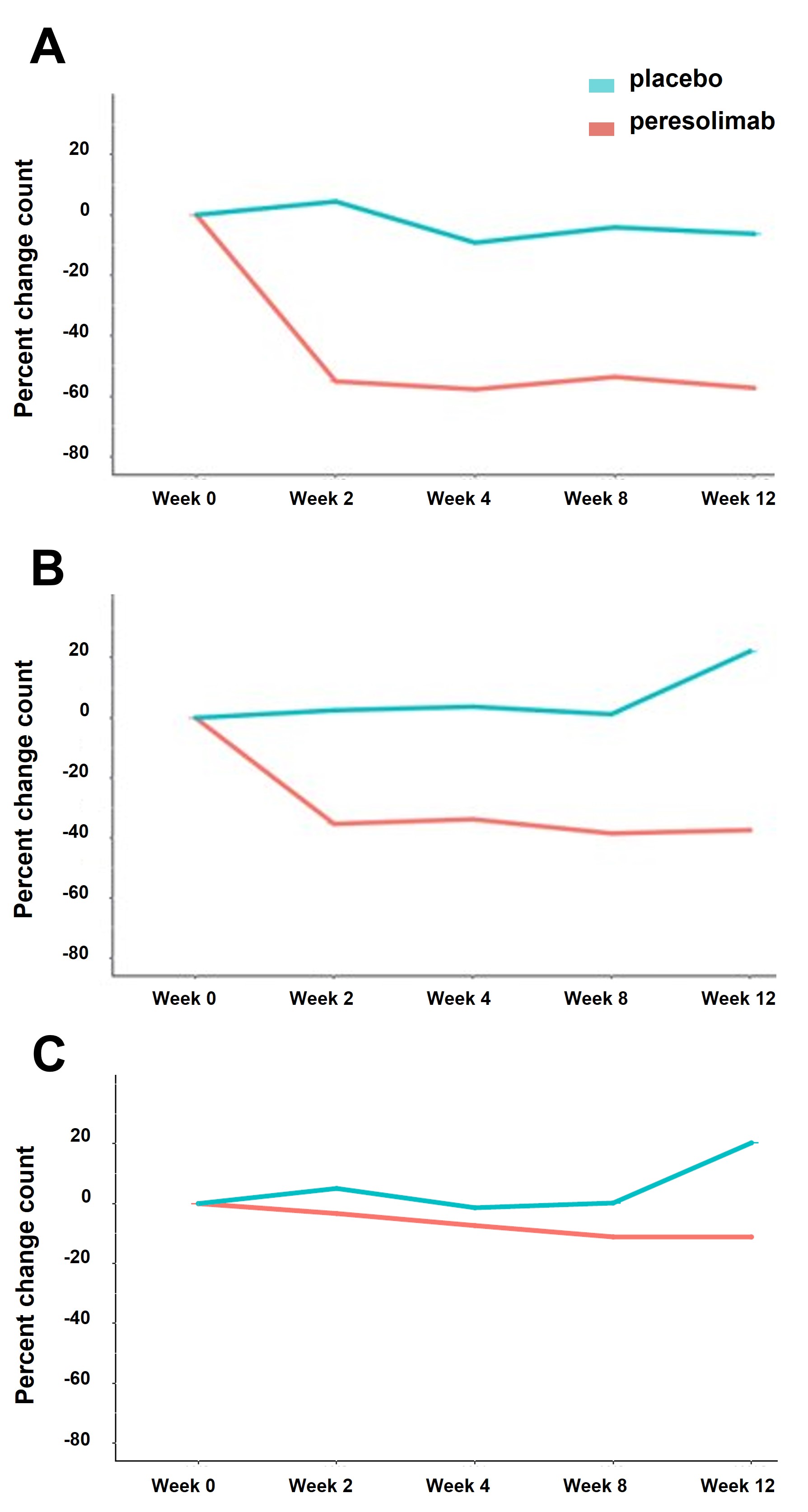
Results: Peresolimab treatment reduced the number of peripheral CD4+ PD-1 high and PD-1 intermediate T cells by 53.6-57.7% and 33.8-38.5%, respectively, relative to baseline from weeks 2 through 12. No meaningful differences were observed for PD-1 low cells; Figure 1. There were no meaningful changes with major circulating cell types (ie, CD3+, CD4+, CD8+ T cells, B or NK cells) following treatment with peresolimab. Analysis of the proteomic data identified multiple pathways that showed differences between peresolimab-treated patients and placebo controls starting at week 8. This included treatment-induced down-modulation of cytokines and chemokines in interleukin signaling pathways, including IL-6, Figure 2. Similar patterns of change were observed for several other immune-related pathways. Overall, pathway analysis suggests that protein levels of peresolimab-treated patients trend toward what was observed in age- and sex-matched healthy controls.
Conclusion: We report here changes in several exploratory biomarkers from patients enrolled in the Phase 2a study of peresolimab in RA patients. A rapid reduction was observed in circulating PD-1 high CD4+ T cells lasting through week 12, but no changes in any of the major lymphocyte subsets were detected with peresolimab treatment. Several molecular pathways that are altered in RA patients, trended towards patterns in healthy subjects with peresolimab treatment as early as week 8, which corresponds to the timing of DAS28-CRP improvements. These results provide insights into the timing and impact of PD-1 inhibition with peresolimab in the treatment of patients with moderate to severe RA.
1. N Engl J Med 2023; 388:1853-1862
To cite this abstract in AMA style:
J. Benschop R, Tuttle J, Emery P, Preuss C, Daniels M, Chan V, Yachi P, Nirula A. Cellular and Proteomic Changes Following Administration of Peresolimab, in a Phase 2a Trial in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/cellular-and-proteomic-changes-following-administration-of-peresolimab-in-a-phase-2a-trial-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cellular-and-proteomic-changes-following-administration-of-peresolimab-in-a-phase-2a-trial-in-rheumatoid-arthritis/