Session Information
Date: Monday, November 13, 2023
Title: (1100–1123) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: To this date, a causal relationship between febuxostat and cardiovascular disease remains controversial as comparison between trials can be challenging and may lead to misleading conclusions especially when facing heterogeneous cardiovascular outcomes. We aimed to compare the cardiovascular outcomes in the most pertinent trials of Febuxostat compared to controls.
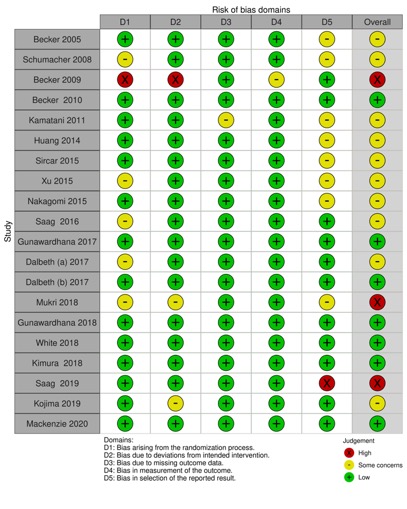
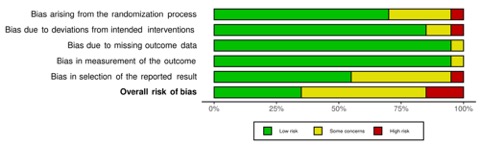
Methods: We searched electronic databases using a PICOS-style approach search strategy of randomized controlled trials on cardiovascular outcomes of Febuxostat in patients with gout or hyperuricemia. We conducted a quality and risk of bias assessment of the included clinical trials. The definition of MACE as well as all reported cardiovascular outcomes were retrieved from every involved trial.
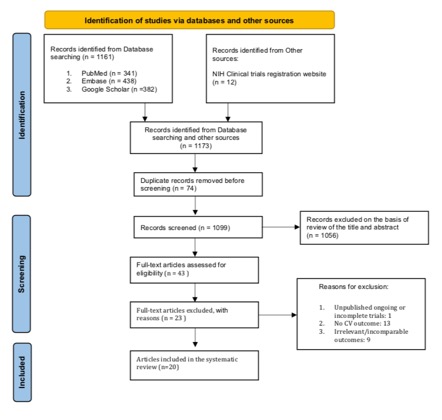
Results: Of the 1173 records identified from all sources, 20 RCTs were included in the analysis. Mean duration of follow-up was 69.7 ± 81.5 weeks and Febuxostat dose ranged from 10 to 240 mg with 80 mg being the most commonly used dosage. Overall, the quality of evidence deriving from all RCTs showed concerns in most studies (65%). Major cardiovascular event (MACE) was defined in 7 of the 20 RCTs (35%) and cardiovascular outcome reporting was very heterogeneous. Overall, data of cardiovascular safety of Febuxostat were reassuring.
Conclusion: Our systematic review showed no alarming increase of cardiovascular mortality and outcomes in Febuxostat treated patients except for the CARES trial in which the credibility of the results is biased by the high rate of drug discontinuation and of most importantly, withdrawal from follow-up. FAST trial results, backed up with real-world data cohort studies, were robust and reassuring with regard to the cardiovascular safety of Febuxostat in most white males with gout including elderly patients. This should lead the regulatory agencies to reconsider the limitations imposed on the use of Febuxostat in patients with non-severe CV burden
NIH: National Institutes of Health; CV: Cardiovascular
To cite this abstract in AMA style:
Ghossan R, Aitisha Tabesh O, Fayad F, Richette P, Bardin T. Cardiovascular Safety of Febuxostat in Patients with Gout or Hyperuricemia: A Systematic Review of Randomized Controlled Trials [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/cardiovascular-safety-of-febuxostat-in-patients-with-gout-or-hyperuricemia-a-systematic-review-of-randomized-controlled-trials/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cardiovascular-safety-of-febuxostat-in-patients-with-gout-or-hyperuricemia-a-systematic-review-of-randomized-controlled-trials/