Session Information
Date: Monday, November 18, 2024
Title: RA – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Preliminary results from the ORAL Surveillance clinical trial showed an increase in the risk of major adverse cardiovascular events (MACE) and malignancies in patients treated with tofacitinib, compared to patients treated with TNF inhibitors (TNFi).
This study aimed to evaluate the safety of JAK inhibitors (JAKi) versus TNFi among patients with rheumatoid arthritis (RA) in a real-world setting.
Methods: Retrospective analysis of 3294 patients with RA included in a national registry of advanced therapies that collects adverse events data from routine clinical practice. A survival analysis was conducted comparing JAKi and TNFi initiated between 2017 and 2022, considering as a completion event the occurrence of specific adverse events (AEs): cardiac, vascular, MACE, and cancer (excluding non-melanoma skin cancer). Survival analysis was performed until the occurrence of the first AE, but excluding subsequent AEs. Hazard ratios (HR) were estimated using multivariate Cox regression model, adjusted by sex, age at treatment start, prior history of outcomes of interest, disease duration, and treatment line. Incidence rates were calculated as the number of events per 1.000 person-years, and Poisson regression was used to compute incidence rate ratios (IRR) comparing JAKi vs TNFi.
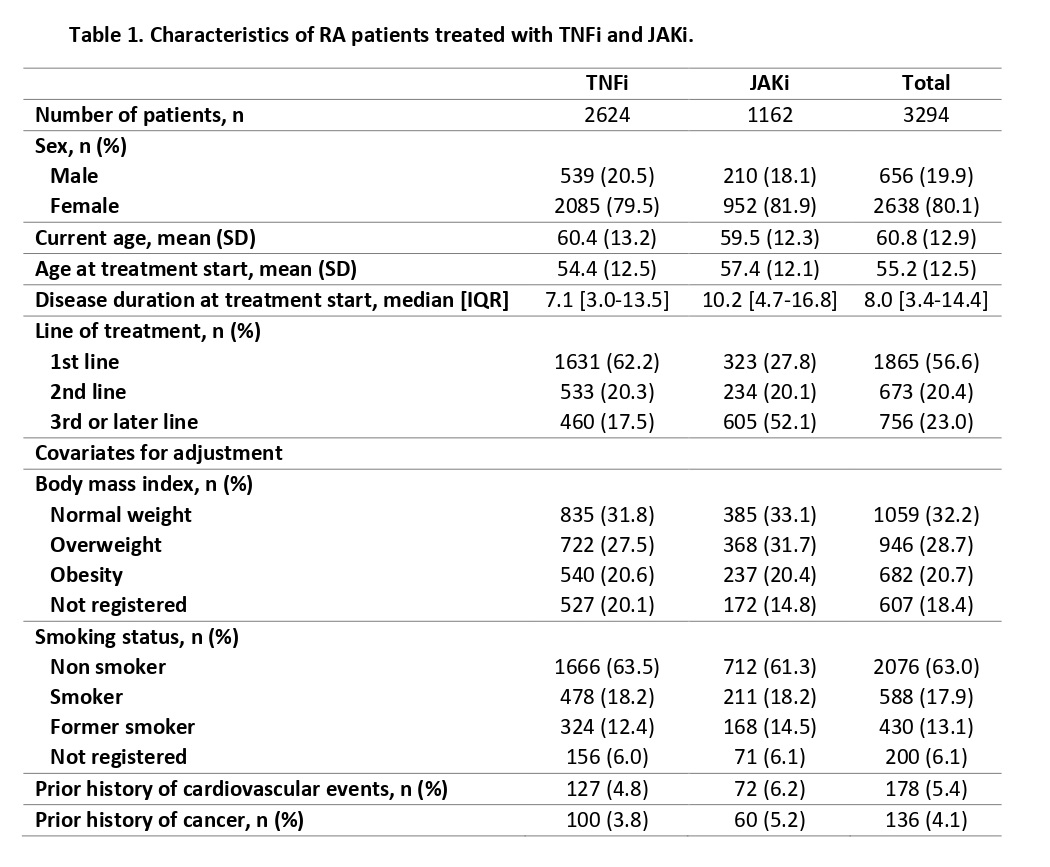
Results: A total of 1162 patients were treated with JAKi and 2624 with TNFi (Table 1). Both treatments were mostly used among females (81.9% vs 79.5%). JAKi were mainly used as third or later line of treatment (52.1%) while TNFi were used as first line of therapy (62.2%). JAKi presented HR greater than 1 for all AEs of interest: cardiac events HR 2.13 (95% confidence interval 1.43-3.18); vascular events HR 2.70 (95% CI 1.55–4.70); MACE HR 1.70 (95% IC 0.48-6.37); and malignancies HR 1.30 (95% CI 0.84-2.00). Table 2 depicts the IR calculated for each treatment group. No statistically significant differences were found among IRR, neither for the treatment groups nor the AE of interest.
Conclusion: Real-world data can be valuable and decisive when assessing the safety of JAKi. Two main findings should be highlighted from this study: on the one hand, the HR for cardiac and vascular events was statistically greater among JAKi compared to TNFi, and significant IRR was found for cardiac AEs. On the other hand, no differences were found in HR nor IRR for MACE and malignancies. Results from this analysis should be interpreted with caution due to the limited exposure time of JAKi and the differences in the patient profile using both treatment types (despite the adjusted regression models).
** IRR adjusted by sex, age at therapy start, disease duration, line of treatment, prior medical history of cancer, and smoking status.
To cite this abstract in AMA style:
Otero-Varela L, Sanchez-Piedra C, Rabadán Rubio E, Sarmiento-Monroy J, Plasencia-Rodríguez C, Sueiro D, Martinez O, Busquets Pérez N, Freire González M, Sánchez-Alonso F, Álvaro-Gracia J, Castrejon I. Cardiovascular and Cancer Safety of JAKi Compared to TNFi in Patients with Rheumatoid Arthritis: Results from a National Registry of Advanced Therapies [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/cardiovascular-and-cancer-safety-of-jaki-compared-to-tnfi-in-patients-with-rheumatoid-arthritis-results-from-a-national-registry-of-advanced-therapies/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cardiovascular-and-cancer-safety-of-jaki-compared-to-tnfi-in-patients-with-rheumatoid-arthritis-results-from-a-national-registry-of-advanced-therapies/