Session Information
Date: Saturday, November 16, 2024
Title: Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The prevalence of cardiac manifestations has not been comprehensively described in anti-synthetase syndrome (ASSD). In the current study, we report the prevalence of cardiac involvement in ASSD patients compared to non-ASSD IIM and other mimicking conditions.
Methods: We utilized the CLASS (Classification Criteria of Anti-Synthetase Syndrome) project database, comprised of physician-reported retrospective data from 92 centers around the world. Cardiac involvement variables included myocarditis, pericarditis, arrhythmias, nonischemic cardiomyopathy, ischemic cardiomyopathy, and pulmonary hypertension (PH). Information on interstitial lung disease (ILD) and lung physiology including forced vital capacity (FVC) and diffusing capacity (DLCO) were also analyzed. Prevalence was calculated among patients with data entered, excluding those with missing data.
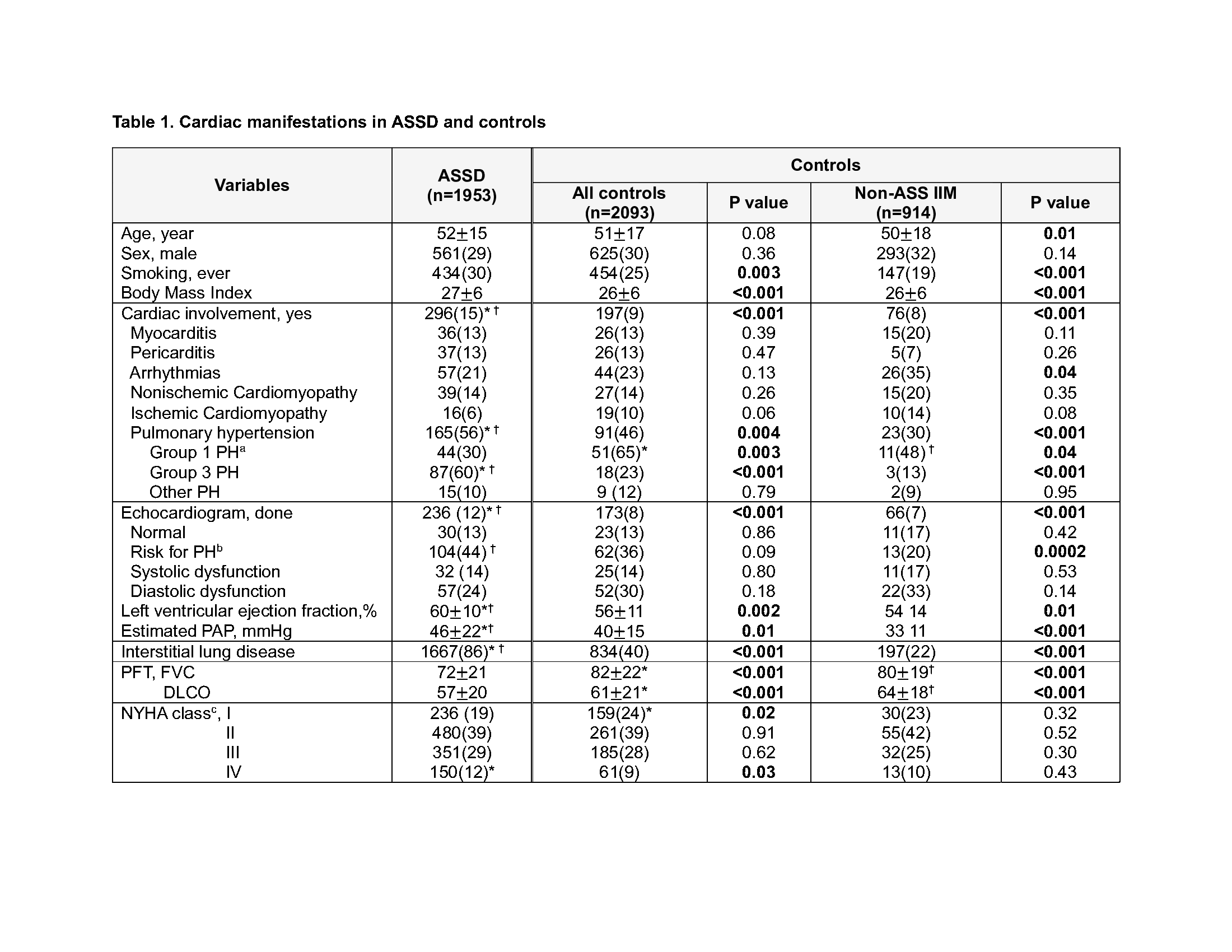
Results: We included 1953 ASSD cases and 2093 controls (914 non-ASSD IIM controls). The comparator group included 44% non-ASSD IIM (28% dermatomyositis, 9% polymyositis, 7% other IIM), 11% rheumatoid arthritis, 11% systemic sclerosis, 8% interstitial pneumonia with autoimmune features, 5% systemic lupus erythematosus, 5% Sjogren’s, and 16% others. The cohort mean age was 52 years and 29% were male which were similar in ASSD and controls (Table 1). ASSD patients had a higher proportion of ever smokers and higher mean body mass index.
Cardiac involvement was reported in 15% of ASSD cases, which was higher compared to all controls and compared to non-ASSD IIM controls (Table 1). The most frequently reported cardiac manifestation in ASSD was PH, with a prevalence of 8.4%. PH in ASSD cases was mostly group 3 PH due to ILD, which was different from controls in which group 1 PH was more common. These results were attributable to the high prevalence of ILD (86%) in ASSD cases vs controls. Pulmonary function test results showed significantly lower FVC and DLCO in ASSD compared to controls.
The prevalences of arrhythmias (2.9%), nonischemic cardiomyopathy (2%), myocarditis (1.8%), pericarditis (1.8%), and ischemic cardiomyopathy (0.8%) in ASSD were not significantly different compared to all controls. Compared to non-ASSD IIM controls, ASSD cases had a significantly lower prevalence of arrhythmias. The most common method for the detection of arrhythmias was electrocardiogram (67%), for myocarditis, cardiac magnetic resonance imaging (58%), and for pericarditis and cardiomyopathy, echocardiogram (31-75%).
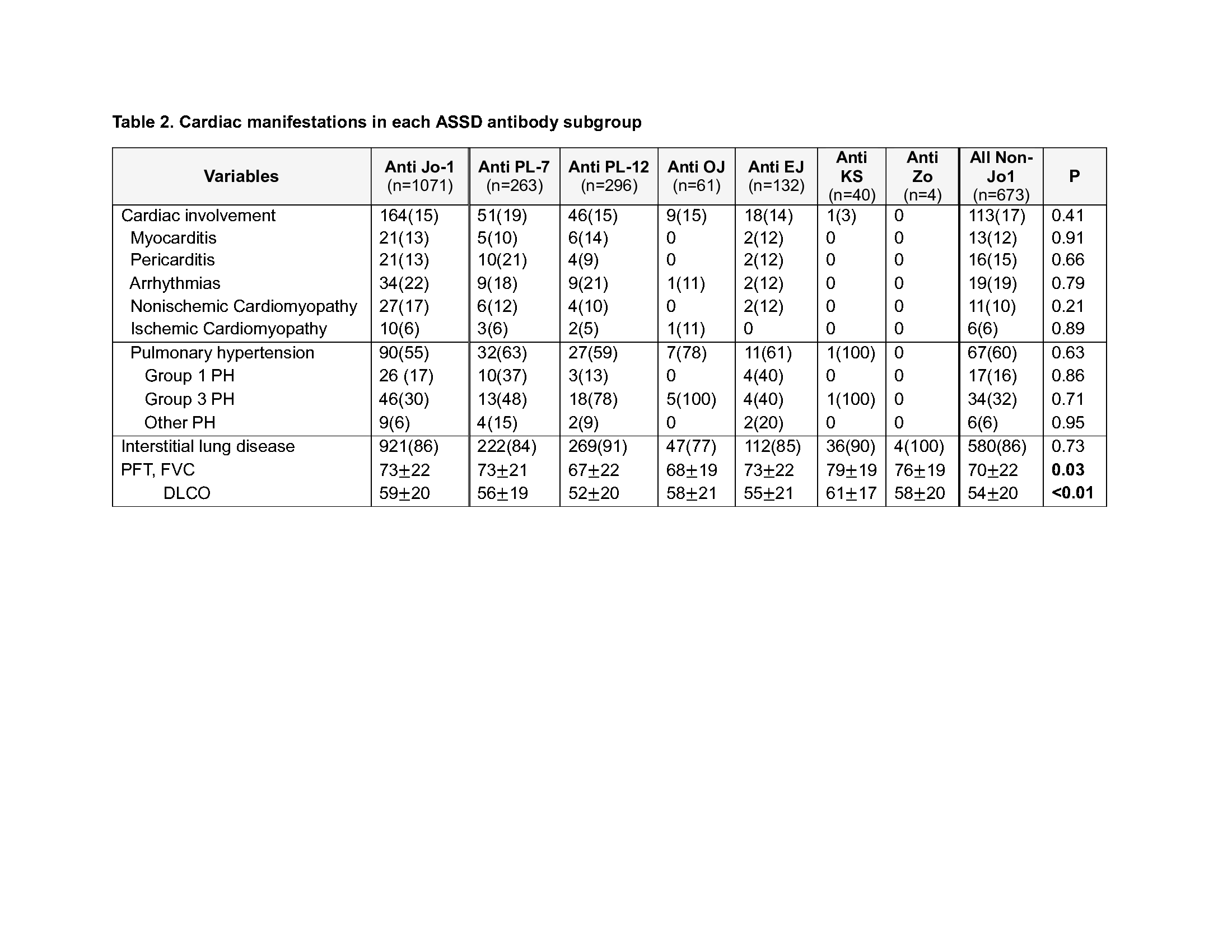
We also analyzed cardiac manifestations in ASSD cases by antibody subgroups. Anti-Jo-1 patients did not have significant differences in their cardiac manifestations compared to non-Jo-1 patients (Table 2). Anti-PL-7 patients had a numerically greater proportion of pericarditis.
Conclusion: The prevalence of cardiac involvement was 15% in patients with ASSD. PH (mostly group 3 with concomitant ILD) was the most common manifestation. Arrhythmias, cardiomyopathy, and pericarditis were seen in less than 3% of ASSD cases, which was not different from controls. The prevalence of cardiac involvement was similar in anti-Jo-1 and non-Jo-1 antibody subgroups.
*marked group with significantly higher value between ASSD vs All control (p<0.05)
†marked group with significantly higher value between ASSD vs non-ASS IIM (p<0.05)
aGroup 1 primary pulmonary artery hypertension, Group 3 secondary pulmonary hypertension due to ILD, Other PH includes pulmonary embolism, due to heart disease including systolic or diastolic dysfunction
bIntermediate or high risk of PH according to 2015 ERS/ESC guidelines
cThe most abnormal or severe New York Heart Association funcitonal class patient ever had
Abbreviation: PAP, pulmonary artery pressure; PFT, pulmonary function test; FVC, forced vital capacity; DLCO, diffusing capacity using carbon monoxide; NYHA, New York Heart Association functional class
Values are n(%) or mean±SD. All % calculated after excluding missing data for any given variable. Prevalence of each variable is calculated within the total of the category
P value calculated comparing Anti Jo_1 patients to all non-Jo_1 patients
To cite this abstract in AMA style:
Bae S, sambataro G, Ventura i, Bozan F, Dourado E, Faghihi-Kashani S, Loganathan A, Rivero Gallegos D, Yoshida A, Zanframundo G, Bonella F, J Corte T, J Doyle T, fiorentino d, Gonzalez-Gay M, Hudson m, Kuwana M, Mammen A, McHugh N, Miller F, Montecucco C, Notarnicola A, Oddis C, Rojas-Serrano J, Schmidt J, Scire C, Gil-Vila A, Werth V, Cavagna L, Aggarwal R. Cardiac Manifestations in Patients with Anti-Synthetase Syndrome: Analysis from the “Classification Criteria for Anti-synthetase Syndrome (CLASS)” Project Database [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/cardiac-manifestations-in-patients-with-anti-synthetase-syndrome-analysis-from-the-classification-criteria-for-anti-synthetase-syndrome-class-project-database/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cardiac-manifestations-in-patients-with-anti-synthetase-syndrome-analysis-from-the-classification-criteria-for-anti-synthetase-syndrome-class-project-database/