Session Information
Date: Monday, November 13, 2023
Title: (1155–1182) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Dermatomyositis (DM), an idiopathic inflammatory myopathy, is a chronic and often debilitating condition characterized by a hallmark skin rash (e.g., Gottron’s sign and heliotrope rash) with perifascicular atrophy and subsequent muscle weakness. The pathogenesis of DM involves dysregulation in signaling of Type I interferon (IFN-I), IFN-ɣ, IL-12, and IL-23. TYK2 and JAK1, are essential for the signaling pathway of these cytokines. Brepocitinib, a selective and potent dual TYK2/JAK1 inhibitor is in development for the treatment of DM and is expected to reduce signaling of these cytokines.A Phase 3 clinical trial of brepocitinib in adults with DM is ongoing (NCT05437263).
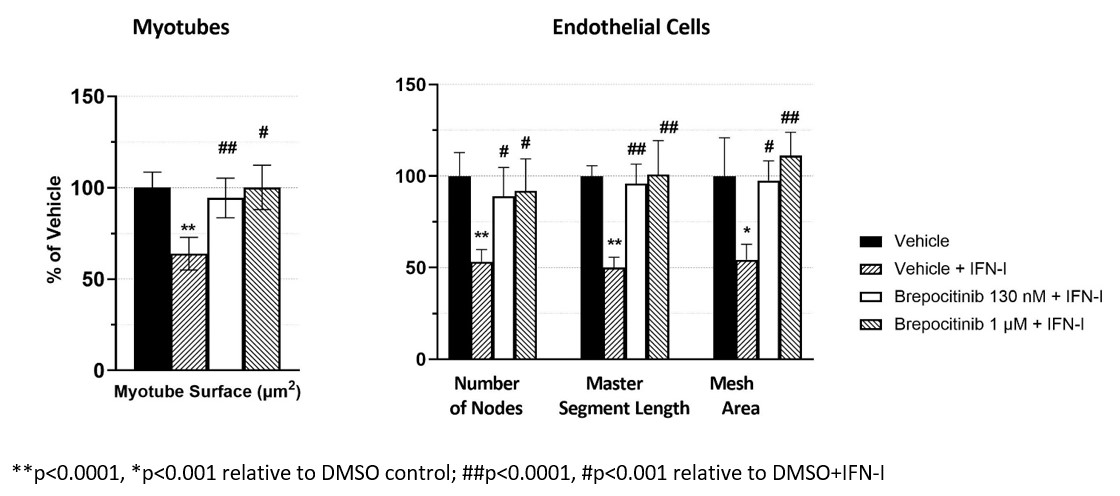
Methods: To evaluate the efficacy of brepocitinib in preventing IFN-I induced pathological changes characteristic of DM, human skeletal muscle myoblasts were cultured and differentiated into myotubes then treated with vehicle control or IFN-I to induce cellular damage. Myotubes were also preincubated for 1 hour with brepocitinib (1 μM or 130 nM, the latter represents the average free plasma concentration after brepocitinib 30 mg QD) prior to IFN-I treatment. Immunofluorescence staining followed by image analysis to determine myosin 4 surface area were conducted after 48 hours of IFN-I treatment. Human dermal microvascular endothelial cells (HMEC-1) were cultured and, once vascular networks were established, cells were treated with IFN-I or vehicle control. Cells were also preincubated for 1 hour with brepocitinib as described above. Under light microscopy 9 hours after treatment, the number of nodes, master segment length, and total mesh area were analyzed. One-way ANOVA followed by Tukey’s multiple comparison post-hoc analyses were performed.
Results: Myosin surface area was reduced by ~40%, relative to vehicle treated control, in myotubes exposed to IFN-I (p< 0.0001). This cytokine induced damage was prevented by brepocitinib preincubation (both 130 nM and 1 μM) with mean myosin surface areas of 96% and 100% of the vehicle control, respectively. The differences between IFN-I treatment alone and brepocitinib were significant (p< 0.0001 [1 μM] and P< 0.001 [130 nM]). Similarly, HMEC-1 exposure to IFN-I significantly reduced the mean number of nodes, mean master segment length, and mean total mesh area by 47 to 50% relative to vehicle control (p< 0.0001 nodes and segments, p< 0.001 mesh area). With brepocitinib preincubation, this damage was prevented with the mean number of nodes, master segment lengths, and total mesh area ranging from 89 to 111% of vehicle control. These differences were statistically different from the IFN-I treatment at both brepocitinib concentrations and all endpoints (p< 0.001 for all conditions).
Conclusion: One of the most debilitating aspects of DM is muscle weakness resulting from perifascicular atrophy leading to significant impacts on quality of life for these patients. We report here the ability of brepocitinib to prevent IFN-I induced damage in both myocytes and microvasculature in culture at clinically relevant concentrations, providing further pharmacologic rationale for brepocitinib in the treatment of DM.
To cite this abstract in AMA style:
Vencovsky J, Feldman J, McConnachie L, Johnson B. Brepocitinib Prevents Type-I Interferon Induced Damage in Cultured Myocytes and Endothelial Cells Indicating a Potential Role in the Treatment of Dermatomyositis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/brepocitinib-prevents-type-i-interferon-induced-damage-in-cultured-myocytes-and-endothelial-cells-indicating-a-potential-role-in-the-treatment-of-dermatomyositis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/brepocitinib-prevents-type-i-interferon-induced-damage-in-cultured-myocytes-and-endothelial-cells-indicating-a-potential-role-in-the-treatment-of-dermatomyositis/