Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: IRL201805 is a novel biologic derived from Binding Immunoglobulin Protein (BiP) that has been developed for the treatment of Rheumatoid arthritis (RA) (P Eggleton, et al, J Cell Mol Med, 2023;27:322-339). A phase I/IIa clinical trial with a single IRL201805 intravenous infusion has demonstrated improvement in Disease Activity Score 28 (DAS28) (B Kirkham, et al, Rheumatology, 2016;55:1993-2000). Moreover, IRL201805 directly interacts with Monocytes, which play a key role in initiation of inflammation and bone erosion in RA. Thus, there is a need to identify the inflammatory pathways that drive the involvement of the myeloid compartment in RA and to how IRL201805 modulates these pathways. Herein, we performed a comprehensive RNA-seq analysis on monocytes from RA patients compared with healthy donors in the presence or absence of IRL201805 to investigate the impact on transcriptional profiles.
Methods: CD14+CD16– monocytes were isolated from peripheral blood mononuclear cells (RA patients with active disease and healthy controls (HC)), and cultured overnight with macrophage-colony stimulating factor (M-CSF). RNAseq was performed on the monocytes collected with or without 24h IRL201805 stimulation.
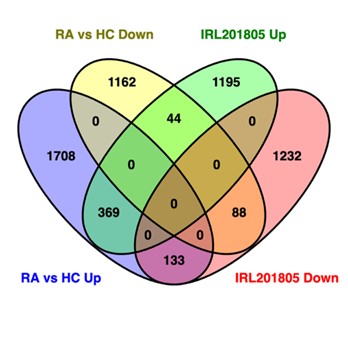
Results: Evaluation of the transcription profiles changes in monocytes from active RA patients and age-gender matched HC in the presence or absence of IRL201805 revealed that untreated monocytes from RA patients had 2210 upregulated differentially expressed genes (DEGs) and 1294 downregulated DEGs when compared to HC cells (adjusted p < 0.05 & absolute log2fold > 0.58). Furthermore, exposure to IRL201805 resulted in the upregulation of 1608 transcripts and downregulated of 1453 in RA monocytes whilst only altering the expression of < 20 genes in cells derived from HC. In RA samples, the upregulated transcripts were associated with cell adhesion and chemotaxis whilst the downregulated transcripts were associated with metabolic processes. To further investigate the effect of IRL201805 upon monocytes, we compared the DEGs in IRL201805 treated RA monocytes (vs untreated RA macrophages) to the DEGs in circulating RA monocytes (vs HC monocytes). This revealed that 133 upregulated DEGs in RA were decreased and 44 downregulated DEGs in RA were increased with IRL201805 treatment. Notably, a larger proportion of DEGs in RA circulating monocytes were altered in the same direction with IRL201805 treatment.
Conclusion: Our data suggests that IRL201805 selectively target transcriptional changes in RA and not healthy monocytes. Notably, IRL201805 downregulates transcripts that are upregulated in RA whilst simultaneously upregulating transcripts that are downregulated in RA. Combined this suggests that IRL201805 has the capacity to ‘reset’ a number of cell-cell and metabolic linked inflammatory pathways in disease-state cells.
In RA monocytes, 44 of the downregulated genes are upregulated with IRL201805 treatment, while upregulated 133 genes were downregulated by IRL201805 treatment.
To cite this abstract in AMA style:
Yamamura Y, Woolcock K, Corrigall V, Ravanetti L, De Alba J, Foulkes R, Eggleton P, Goodyear C. Biologic IRL201805 Drives Differential Cell-contact and Metabolism Transcriptional Profiles in Monocytes from RA Patients Compared to Healthy Donors [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/biologic-irl201805-drives-differential-cell-contact-and-metabolism-transcriptional-profiles-in-monocytes-from-ra-patients-compared-to-healthy-donors/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/biologic-irl201805-drives-differential-cell-contact-and-metabolism-transcriptional-profiles-in-monocytes-from-ra-patients-compared-to-healthy-donors/