Session Information
Date: Tuesday, November 19, 2024
Title: Abstracts: Miscellaneous Rheumatic & Inflammatory Diseases II
Session Type: Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Targeting B cells and plasma cells is a key therapeutic strategy in autoimmune disease
(AID). Therapy with CD19-targeted chimeric antigen receptor (CAR) T-cells and bi-specific T cell engagers (BITEs) demonstrated efficacy in treatment-resistant AID1,2.
However, in some patients, disease may be anchored in long-lived plasma cells in the bone marrow expressing BCMA but not CD19. For instance, SS-A/Ro, PM-Scl and PL-7 autoantibodies remain present after CD19-CAR T-cell therapy.
Teclistamab is a BITE that engages T-cells via CD3 and targets plasma cells via BCMA. We hypothesized that teclistamab may be effective in severe AID even after failure of conventional B cell depletion.
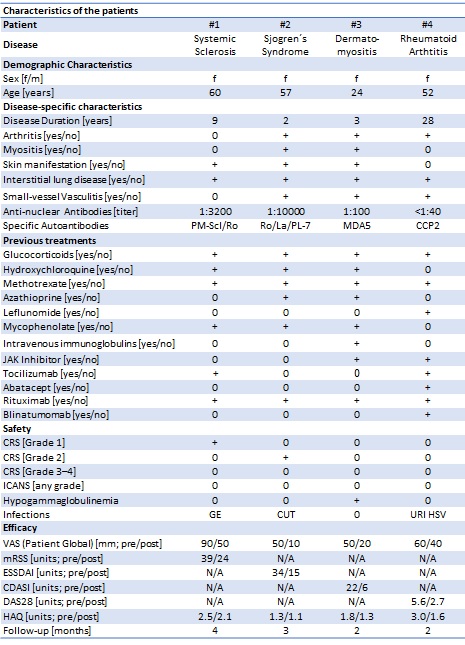
Methods: We treated 4 patients with multi-drug-resistant AID with teclistamab under compassionate use (Table 1). Pt #1 had systemic sclerosis with calcinosis cutis and interstitial lung disease (ILD). Pt #2 had Sjogren´s syndrome with arthritis, enthesitis, myositis, inflammatory rash and ILD. Pt #3 had progressive MDA5+ dermatomyositis with arthritis, myositis, inflammatory rash, digital ulcers and ILD. Pt #4 had seropositive rheumatoid arthritis. All patients had failed >5 immunosuppressants including rituximab.
Teclistamab was dosed-up in an in-patient setting: day 1 (0.06 mg/kg), day 3 (0.3 mg/kg) and day 5 (1.5 mg/kg) and repeated once (1.5 mg/kg) within one month. All immunosuppressants were stopped, except methotrexate in pt #4 and low-dose glucocorticoids in pt #2–4. Follow-up ranged from 6–16 weeks.
Results: Teclistamab was safe and well tolerated. Mild cytokine release syndrome (grade 1: N=1, grade 2: N=1), and mild infections (gastroenteritis, pt #1; cutaneous infection, pt #2; herpes labialis, upper respiratory tract infection, pt #4) occurred.
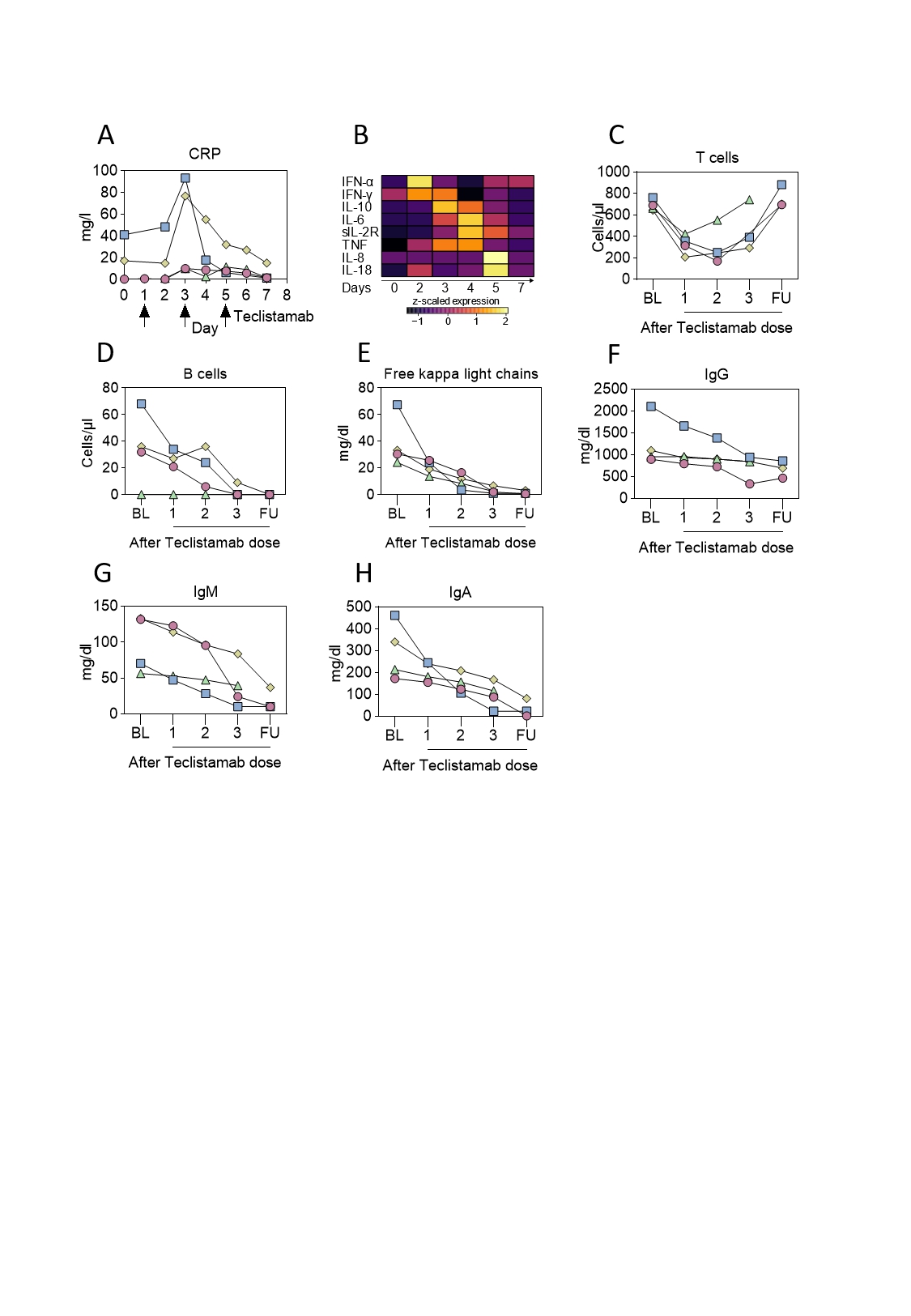
Teclistamab led to T cell engagement as evident by transient elevation in C-reactive protein and inflammatory cytokines, which peaked between day 2 and day 5 (Fig. 1A-B). T cell engagement led to transient T cell consumption and rapid recovery (Fig. 1C).
Circulating B cells were depleted (Fig. 1D) and free light chains were reduced (Fig. 1E), documenting the successful targeting of plasma cells. Immunoglobulin levels decreased (Fig. 1F-H). ANA, PM-Scl-75, PM-Scl-100, RF and MCV antibodies decreased, SS-A/Ro, SS-B/La and PL-7 remained stable.
After 12 weeks, B cells had recovered in pt #1. High dimensional flow cytometry revealed a depletion of class-switched memory B cells and plasmablasts, which were replaced by nonclass-switched, IgD positive naïve B cells.
Teclistamab improved disease activity in all four patients. In pt #1, skin disease (modified Rodnan skin score) improved from 39 to 28. In pt #2, Sjogren´s syndrome activity (ESSDAI) improved from 34 to 15. In pt #3, skin inflammation (CDASI) improved from 22 to 6. In pt #4, arthritis (DAS28-CRP) improved from 5.9 to 2.7.
Conclusion: Our results suggest that targeting the plasma cells via BCMA-targeted BITEs is safe and effective in AID.
References:
1. Müller F, Taubmann J et al. CD19 CAR T-Cell Therapy in Autoimmune Disease – A Case Series with Follow-up. N Engl J Med. 2024;390(8):687-700.
2. Bucci L, Hagen M et al. Bispecific T cell engager therapy for refractory rheumatoid arthritis. Nat Med (2024).
CUT=cutaneous infection, URI=upper respiratory infection.
To cite this abstract in AMA style:
Bucci L, Hagen M, Boeltz S, Noethling D, Rothe T, Raimondo M, Tur C, Wirsching A, Wacker J, Bozec A, Schett G, Grieshaber-Bouyer R. BCMA-targeted Bispecific T Cell-engager Therapy of Autoimmune Disease [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/bcma-targeted-bispecific-t-cell-engager-therapy-of-autoimmune-disease/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bcma-targeted-bispecific-t-cell-engager-therapy-of-autoimmune-disease/