Session Information
Date: Sunday, November 7, 2021
Title: Pediatric Rheumatology – Clinical Poster II: SLE, JDM, & Juvenile Scleroderma (0764–0785)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Juvenile dermatomyositis (JDM), the most common inflammatory myopathy of children, is rare, with an estimated incidence of 2-4 in 1 million children. Given the challenges of studying JDM, large observational cohort studies are invaluable resources for disease characterization, long-term monitoring, and comparative effectiveness studies, to ultimately improve the care and outcomes of children with JDM.
The Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry, designed a longitudinal inception cohort that includes the systematic data collection, including patient reported outcomes, planned over 10 years.
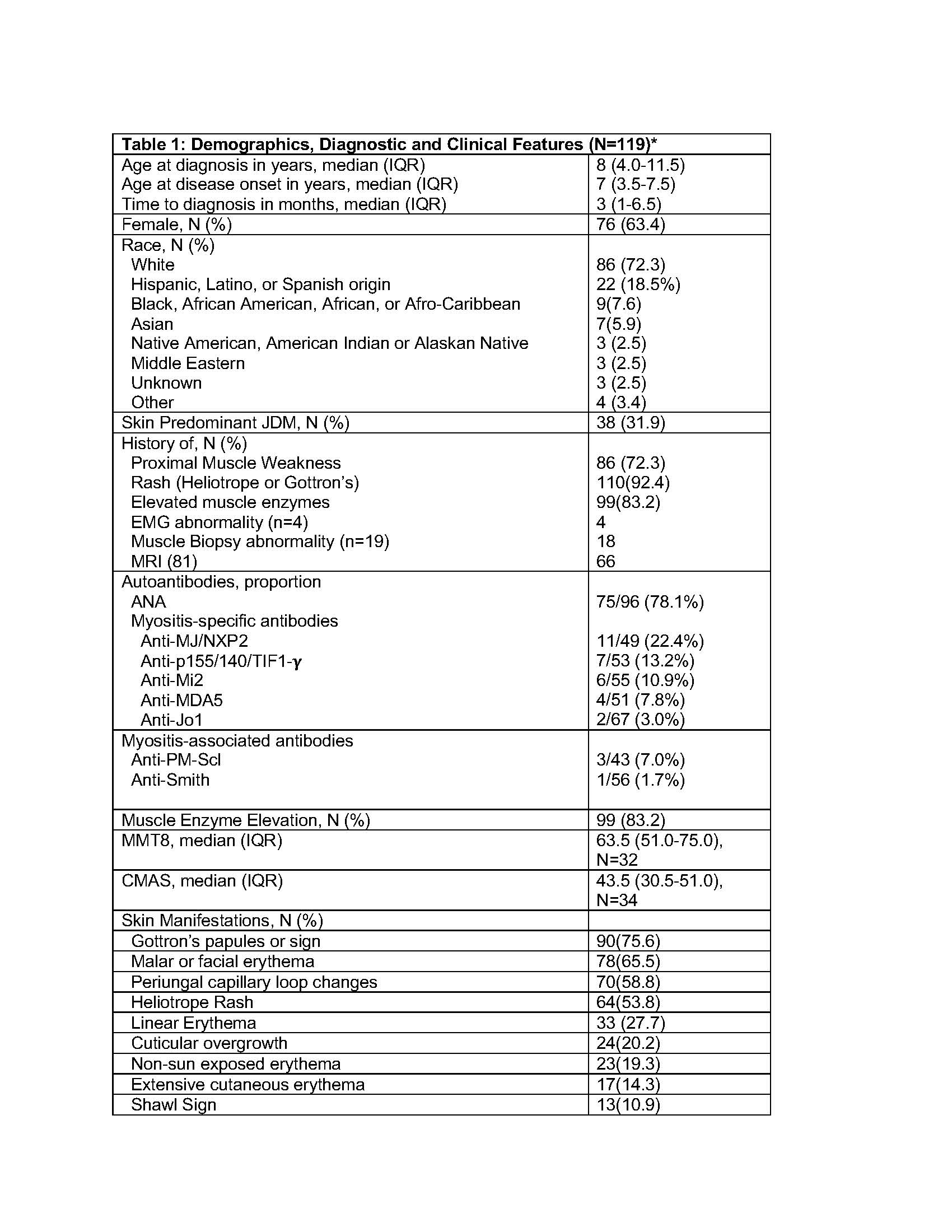
Here, we describe the baseline patient demographics, disease characteristics, initial assessments, patient/parent-reported outcomes and treatments for an inception cohort of children with JDM enrolled to the registry in the first year.
Methods: The CARRA Registry began enrollment of JDM subjects in January 2019. Eligible subjects were < 18 years of age at disease onset and diagnosed with JDM based on clinical judgement of the treating rheumatologist. Enrolled subjects had disease for < 6 months and treatment naïve or treated with systemic therapy for < 12weeks. This Registry is approved by Duke University Institutional Review Board (IRB) and each participating site. Consent/assent was obtained from eligible participants by the local investigators.
Results: 119 JDM subjects were enrolled from 41 sites. Most were female (63.4%), and white (72.3%) with a median diagnosis age 8.0 years (IQR 4.0-11.5), and median age of disease onset 7.0 years (IQR 3.5-7.5) (Table 1). They had characteristic rashes (92.4%), elevated muscle enzymes (83.2%), physician global score 4.0 (IQR 2.5-5.0) and manual muscle testing score 63.5 (IQR 51.0-75.0). Calcinosis (3%) and interstitial lung disease (< 1%) were uncommon.
CHAQ results (median 0.75, IQR 0.03-1.875), and patient/parent global assessments of disease activity (median 3, patient IQR: 1.75-5.25; parent IQR: 1-7) showed mild-moderate disability. PROMIS® Pediatric Global Health 7 scores, PROMIS measures for pain interference, physical function scores for mobility, and upper extremity function were commonly worse than 95% of the general pediatric population based on published normative data (Table 2).
Approximately half of subjects, 54.6% (n=64), were treated according to a Consensus Treatment Plans (CTP) (Figure 1).
Conclusion: In its first year 119 JDM patients were successfully enrolled into the CARRA Registry. They were similar to historic cohorts, with a trend toward earlier time to diagnosis and milder disease. PROs were also collected successfully, and our findings reveal moderate to severe patient and parent-reported disease impact. Published CTPs were also used to treat over half of the enrolled patients, suggesting that pragmatic trials may be feasible in the CARRA Registry.
This growing registry provides infrastructure to advance future clinical research and paired specimens in the CARRA biorepository will enable future translational research. Together, these efforts enhance trial readiness for future therapeutic studies, including comparative effectiveness trials which will help to advance care and outcomes for JDM.
To cite this abstract in AMA style:
Neely J, Ardalan K, Huber A, Kim S. Baseline Characteristics and Patient Reported Outcomes from a Juvenile Dermatomyositis Registry Inception Cohort [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/baseline-characteristics-and-patient-reported-outcomes-from-a-juvenile-dermatomyositis-registry-inception-cohort/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baseline-characteristics-and-patient-reported-outcomes-from-a-juvenile-dermatomyositis-registry-inception-cohort/