Session Information
Date: Monday, November 8, 2021
Title: Systemic Sclerosis & Related Disorders – Clinical Poster II (1364–1390)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Autologous hematopoietic stem cell transplantation (AHSCT) is now established as a preferred standard of care treatment for patients with severe scleroderma with internal organ involvement (SSc). Two large randomized clinical trials of AHSCT vs. monthly cyclophosphamide (CYC) used CD34+ HSC enrichment to deplete immune cells from the autologous peripheral blood stem cell (PBSC) graft. One trial used total body irradiation (TBI)/CYC/anti-thymocyte globulin (ATG) conditioning regimen1, the other used CYC/ATG (no TBI)2. After these trials, due to reporting issues, many SSc patients given HSCT received unmanipulated PBSC (no CD34+ enrichment). We sought to (1) update the outcomes of AHSCT for SSc in North America in a larger contemporary cohort and (2) examine the importance of patient factors and transplant regimens for overall survival (OS) and progression free survival (PFS).
Methods: A questionnaire for SSc-specific health status before and after AHSCT was created for patients reported to the Center for International Blood & Marrow Transplantation Research. Participating HSCT/rheumatology sites completed questionnaires for each SSc patient transplanted between 2000 to 2020, by conducting medical records review/ telephone interviews in early/mid-2021. Health status, physical function, toxicities, DMARD use and major medical events were recorded. Event endpoints included time to death, initiation of unplanned DMARD, organ failure/transplant, supplemental oxygen, new diagnosis of pulmonary arterial hypertension, enteral feeding tube, total parenteral nutrition, or subsequent HSCT.
Results: 104 patients with SSc were reported to the registry and provided SSc-specific health data. All patients had skin involvement, 56% with lung, 29% lung with other organ, 3% with kidney/GI, and 13% with skin only SSc. Conditioning with TBI/CYC/ATG was in 45%, CYC/ATG was in 55%. CD34+ enrichment of PBSC was used in 39%, 61% without enrichment. 64% had AHSCT between 2015 to 2020. Median follow-up was 41 (range, 5-222) months. Median age at AHSCT was 48 (range, 14-74) years (yr). For the entire cohort of 104 patients (Fig.1), the 5-yr OS was 91%, (95% confidence interval, 84-95). The 5-yr PFS was 56% (45-67). Fig.2, the 5-yr OS with TBI/CYC/ATG was 87% (75-96) and CYC/ATG 94% (86-99) (P-value = 0.16). The corresponding 5-yr PFS were 60% (45-75) and 51% (33-68) (P = 0.92). Fig.3, the 5-yr. OS for the cohort given CD34+ enriched PBSC was 96% (85-100) and unmanipulated PBSC was 87% (77-95) (P = 0.28). The 5-yr. corresponding PFS were 67% (49-83) and 50% (36-64) (P = 0.13). ATG source (horse vs. rabbit) did not affect OS/PFS. Age, examined as a predictor for OS/PFS, was not significant. In multivariate analysis, no tested covariates were significant for OS or PFS.
Conclusion: The OS and PFS benefit of AHSCT for patients with severe SSc was confirmed in this 5-year retrospective, real-world, non-randomized analysis and was independent of the patient factors or transplant regimens analyzed. While the data suggested use of CD34+ enrichment of PBSC for AHSCT improved PFS for SSc patients, study of a larger cohort for a longer time is needed to evaluate significance.
References 1Sullivan, NEJM 2018; 378:35-47 2van Laar, JAMA 2014; 311:2490-98
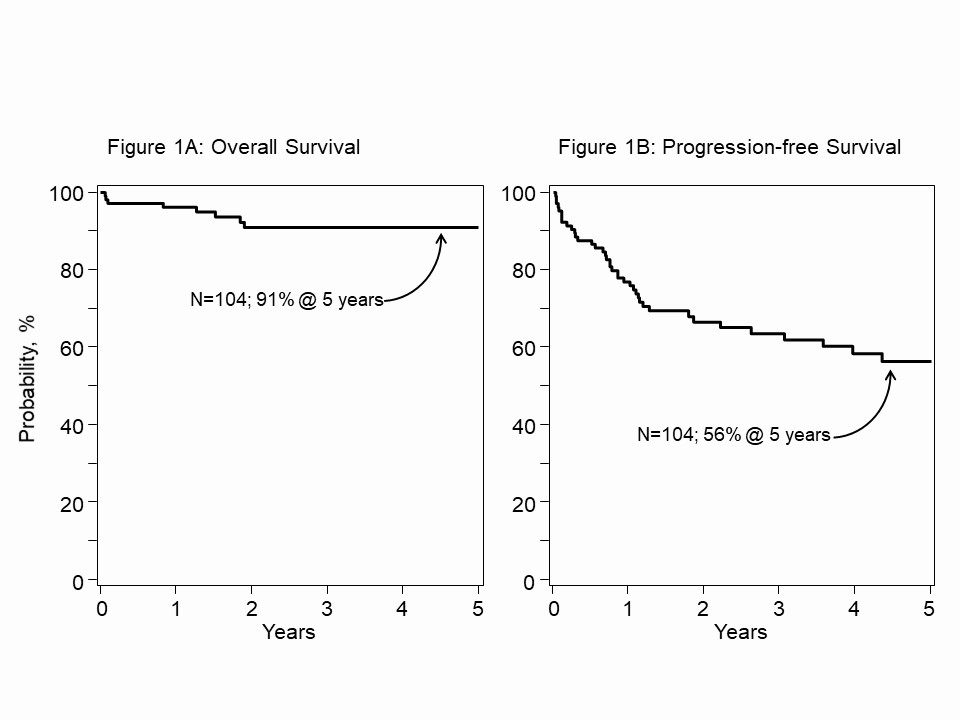 Figure 1. A. 5-year overall survival (OS) for 104 SSc registry patients with AHSCT between 2000 and 2020. B. Corresponding progression-free survival (PFS). Kaplan-Meier (KM) estimate.
Figure 1. A. 5-year overall survival (OS) for 104 SSc registry patients with AHSCT between 2000 and 2020. B. Corresponding progression-free survival (PFS). Kaplan-Meier (KM) estimate.
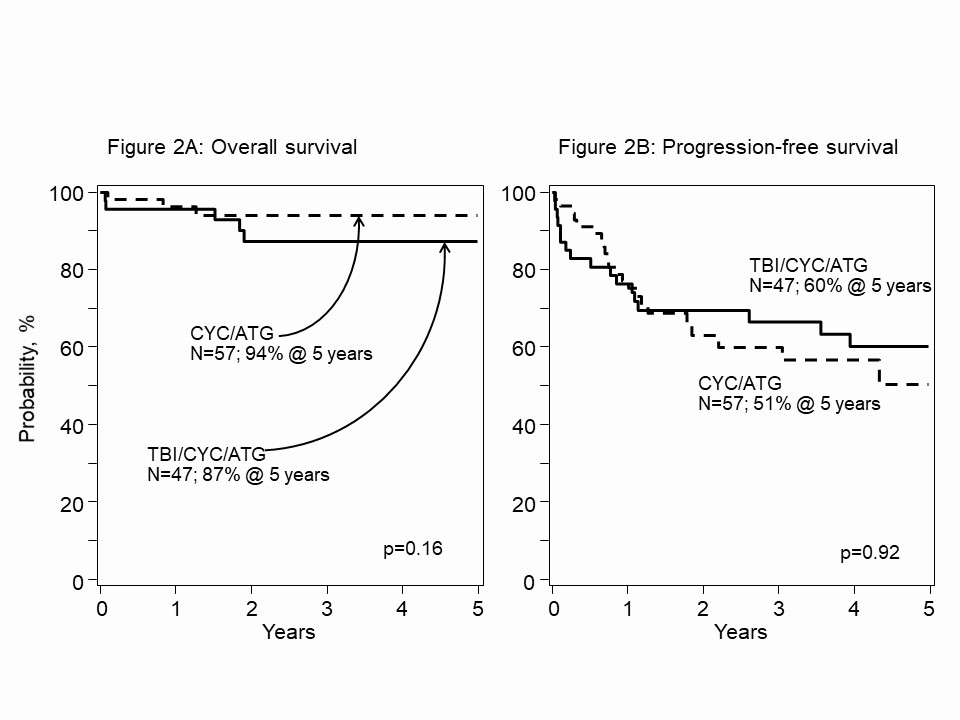 Figure 2. A. 5-yr. OS after AHSCT for SSc, non-randomized, registry patients conditioned with TBI/CYC/ATG vs. CYC/ATG, 87% and 94%, P= 0.16. B. Corresponding 5-yr. PFS, 60% and 51%, P=0.92, log-rank test.
Figure 2. A. 5-yr. OS after AHSCT for SSc, non-randomized, registry patients conditioned with TBI/CYC/ATG vs. CYC/ATG, 87% and 94%, P= 0.16. B. Corresponding 5-yr. PFS, 60% and 51%, P=0.92, log-rank test.
 Figure 3. A. 5-yr. OS after AHSCT for SSc, non-randomized, registry patients with PBSC CD34+ enriched vs. unmanipulated, 96% and 87%, P=0.28. B. Corresponding 5- yr. PFS 67% and 50%, P=0.13, log-rank test.
Figure 3. A. 5-yr. OS after AHSCT for SSc, non-randomized, registry patients with PBSC CD34+ enriched vs. unmanipulated, 96% and 87%, P=0.28. B. Corresponding 5- yr. PFS 67% and 50%, P=0.13, log-rank test.
To cite this abstract in AMA style:
Georges G, Sullivan K, Khanna D, Mei M, Mayes M, Furst D, Wener M, Kafaja S, Long G, Sanchorawala V, Jamani K, Pawarode A, Masurekar A, Lam B, Nash R, Trojanowski M, Hosing C, Clements P, Helms T, McLaughlin B, Griffith L, Hebert K, Eapen M, Storek J, Atkins H. Autologous Hematopoietic Stem Cell (HSC) Transplantation for Systemic Sclerosis, North American Registry: Updated Outcomes and the Impact of CD34+ HSC Enrichment [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/autologous-hematopoietic-stem-cell-hsc-transplantation-for-systemic-sclerosis-north-american-registry-updated-outcomes-and-the-impact-of-cd34-hsc-enrichment/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/autologous-hematopoietic-stem-cell-hsc-transplantation-for-systemic-sclerosis-north-american-registry-updated-outcomes-and-the-impact-of-cd34-hsc-enrichment/
