Session Information
Date: Monday, November 8, 2021
Title: Systemic Sclerosis & Related Disorders – Clinical Poster II (1364–1390)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Systemic sclerosis (SSc) is a clinically heterogenous disease typically characterized by a positive ANA (ANA+), and prototypical antibodies including anti-centromere, anti-topoisomerase, and anti-RNA polymerase III antibodies which each carry specific clinical associations. A subset of SSc patients, however, lack these prototypic SSc related autoantibodies (triple negative), and have been poorly characterized largely due to lack of clinical testing for additional autoantibodies. The purpose of this study was to identify the prevalence of autoantibodies present in ANA+ triple negative patients and assess their clinical correlations.
Methods: Patients with ANA+ and triple negative for prototypic SSc associated antibodies were selected from the scleroderma biorepositories at University of Rochester (UR) and Northwestern University (NU). Demographic and clinical data was obtained to identify specific disease outcomes and associations. Sera were screened for ANA by indirect immunofluorescence testing on HEp-20-10 slides with respect to the intensity, pattern, and titer through EUROPattern microscope. Autoantibody identification was performed using the EUROLINE SSc and Autoimmune Inflammatory Myopathies profiles (EUROIMMUN, Lubeck, Germany).
Results: Of the 280 SSc patient sera queried from UR and NU, 57 were identified as ANA+ and triple negative using clinical testing, and 40 were confirmed to have this status after EUROLINE testing. Of these 40 patients, 53% had limited cutaneous SSc, 83% of patients were female, 75% Caucasian, mean age 53 ± 14.5 years, with disease duration 9 ± 9.7 years. Patients had an average MRSS 7.6 ± 6.8, 19 (47.5%) patients had digital ulcers, 24 (60%) had interstitial lung disease (ILD) with an average FVC of 79 ± 20.6 percent predicted and DLCO 62 ± 19.5 percent predicted, and 6 (15%) had pulmonary hypertension. Of the ANA patterns, the majority of patients had mixed speckled/nucleolar patterns (42.5%) or speckled (30%). Of 29 autoantibodies tested, the most prevalent were Ro-52 (50%), Th/To (40%), MDA5 (35%), SAE1 (27.5%), PM-75 (25%), fibrillarin (25%) (Table 1). Ro-52 was associated with ILD (RR 2.67, 95% C.I. 1.51-5.29, p< 0.001) and elevated CK (RR 2.64, 95% C.I. 1.11-6.96, p< 0.05), PM-75 was associated with digital ulcers (RR 2.18, 95% C.I. 1.17-3.85, p< 0.05), and Mi-2b was associated with increased MRSS (RR 4.00, 95% C.I. 1.25-11.75. p< 0.05).
Conclusion: Patients defined as ANA+ triple negative have equal prevalence of lcSSc and dcSSc, and high prevalence of digital ulcers and ILD. These patients have a variety of autoantibodies which are not typically clinically assessed, and each has important clinical associations, particularly Ro-52 which is present in 50% of patients and strongly associated with ILD. ANA+ triple negative represents a unique and heterogenous patient population which should be further characterized in larger SSc cohorts.
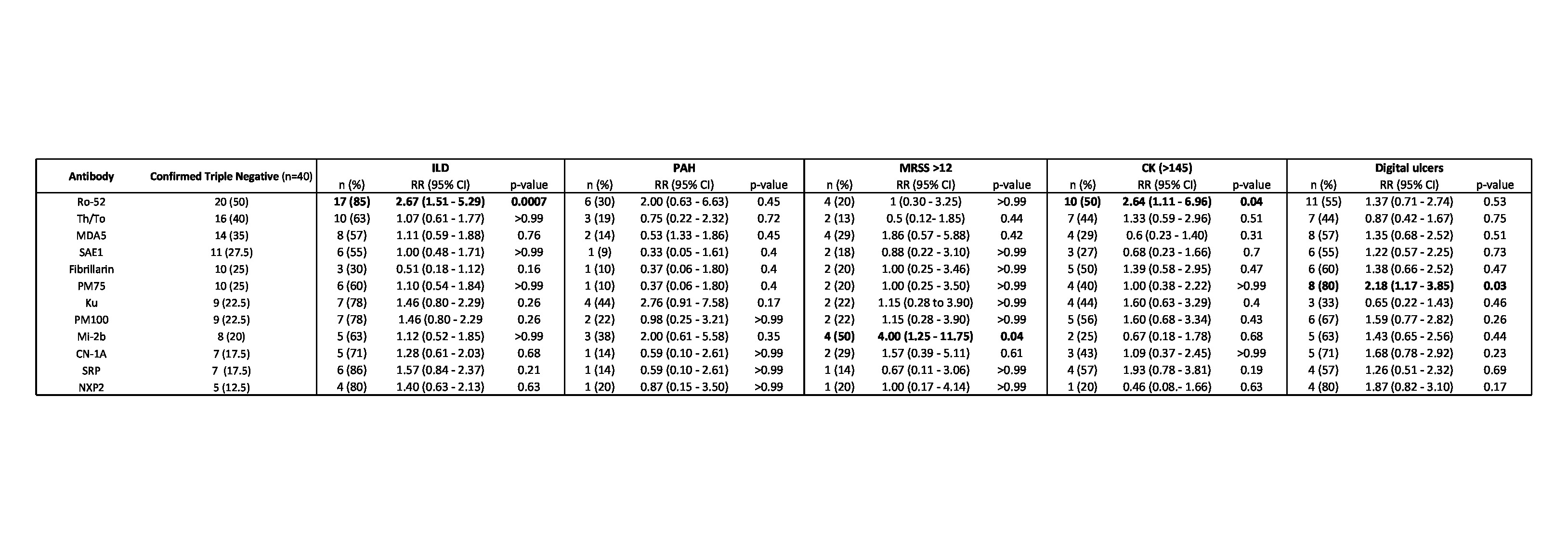 Table 2. Antibody prevalence as measured by immunoblot. Prevalence of scleroderma and myositis specific antibodies in the ANA positive triple negative cohort, antibodies with a prevalence < 10% are not shown. Associations with clinical variables (interstitial lung disease (ILD), pulmonary arterial hypertension (PAH), modified rodnan skin score (MRSS), creatine kinase (CK), and digital ulcers) were ascertained using a Fisher's exact test. Percentage prevalence of each clinical feature is calculated based on antibody prevalence. Statistically significant results (p < 0.05) are highlighted.
Table 2. Antibody prevalence as measured by immunoblot. Prevalence of scleroderma and myositis specific antibodies in the ANA positive triple negative cohort, antibodies with a prevalence < 10% are not shown. Associations with clinical variables (interstitial lung disease (ILD), pulmonary arterial hypertension (PAH), modified rodnan skin score (MRSS), creatine kinase (CK), and digital ulcers) were ascertained using a Fisher's exact test. Percentage prevalence of each clinical feature is calculated based on antibody prevalence. Statistically significant results (p < 0.05) are highlighted.
To cite this abstract in AMA style:
Kruzer K, Marangoni R, Heckler I, Elhage A, John V, Hinchcliff M, Carns M, Aren K, Wielgosz A, Nuzzo M, Venkataraman I, Korman B. Assessment of Autoantibodies and Clinical Associations in SSc Patients with ANA Positivity & Negative for Prototypic Autoantibodies [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/assessment-of-autoantibodies-and-clinical-associations-in-ssc-patients-with-ana-positivity-negative-for-prototypic-autoantibodies/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessment-of-autoantibodies-and-clinical-associations-in-ssc-patients-with-ana-positivity-negative-for-prototypic-autoantibodies/
