Session Information
Date: Tuesday, October 28, 2025
Title: (1855–1876) Systemic Sclerosis & Related Disorders – Basic Science Poster II
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Antinuclear autoantibodies (ANA) are robust biomarkers for the diagnosis and prognosis of systemic sclerosis (SSc), but their role in pathogenesis is still uncertain. Recent evidence showed that purified IgG from SSc patients alter the molecular signatures of key effector cells (fibroblasts [FB] and endothelial cells [EC]) in a serotype-dependent manner, and that ANA might interact with intracellular antigens. This study aims to evaluate ANA’s ability to penetrate cells, understand the internalization mechanisms and determine intracellular interactions.
Methods: We employed a panel of antibodies (Ab) comprising total purified IgG from anti-topoisomerase I positive (IgG-ATA⁺) and anti-centromere positive (IgG-ACA⁺) patients, alongside IgG from healthy controls (IgG-HC). In addition, we utilized polyclonal SSc ATA (poly-ATA, TopoGEN®) and fluorochrome-conjugated poly-ATA antibodies (fluo-poly-ATA). Following a 72-hour culture and 24-hour serum deprivation, FB and EC were incubated with the aforementioned antibodies for 5 minutes to 6 hours. For fixed-cell imaging, cells were washed post-incubation, fixed, permeabilized, and stained using anti-human IgG antibodies. For live-cell imaging, fluo-poly-ATA was added to the cultures, and time-lapse experiments were performed using confocal microscope. Confocal images were obtained using a LSM 980 Airyscan 2 confocal microscope (Zeiss) and analyzed with Imaris 10.2 software (Oxford Instruments).
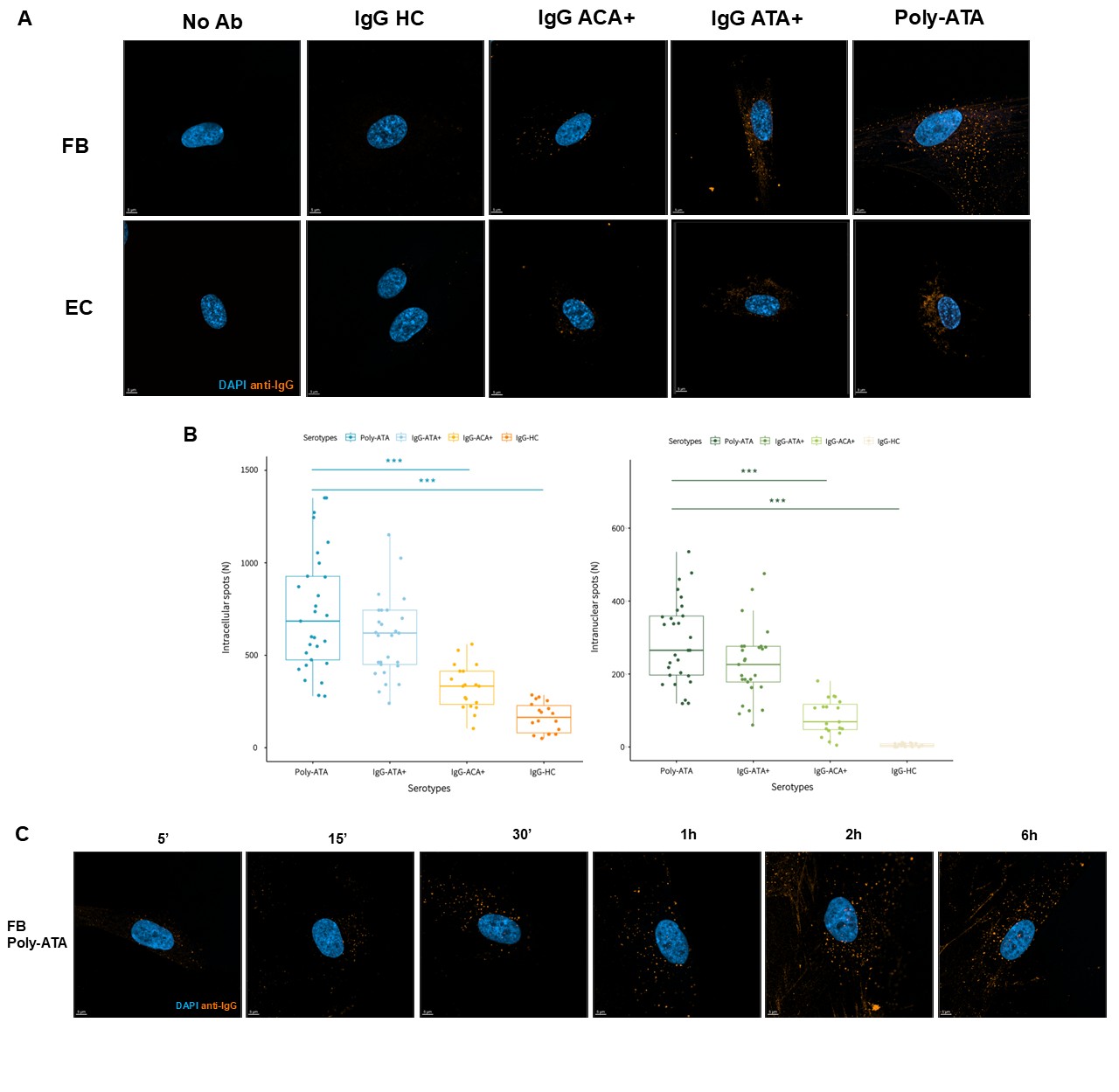
Results: Following 1-hour of Ab incubation, intracellular fluorescent spots were detected in cytoplasmic and nuclear compartments of FB and EC exposed to total IgG from SSc patients and poly-ATA. These spots were more numerous in cells treated with IgG-ATA⁺ and poly-ATA compared to IgG-ACA+. In contrast, while some fluorescence was detected in cells incubated with IgG-HC, nuclear localization was minimal to absent under this condition (Figure 1A and B). Using poly-ATA, fluorescent signal appeared as early as 5 minutes post-incubation and increased progressively up to 6h (Figure 1C). Live-cell microscopy confirmed the rapid cellular uptake and nuclear penetration of fluo-poly-ATA. To verify nuclear localization of ANA, Z-stack confocal imaging was performed with 0.2 μm optical sections to generate three-dimensional cell reconstructions. These analyses confirmed nuclear localization of poly-ATA and fluo-poly-ATA both in FB and EC (Figure 2A). Co-immunostaining with topoisomerase-1 revealed 28% and 31% colocalization with IgG-ATA⁺ and poly-ATA, respectively, while CENP-B protein exhibited 22% colocalization with IgG-ACA⁺ (Figure 2B). To investigate the mechanism of internalization, endocytic pathway inhibition assays were conducted. Pretreatment of FB with a clathrin-mediated endocytosis inhibitor significantly reduced intracellular fluorescence, whereas caveolin pathway inhibitor had no observable effect (Figure 2C).
Conclusion: SSc ANA can penetrate cells and reach their target antigen in vitro. A clathrin-mediated endocytosis mechanism for ANA internalization is suggested. Further mechanistic and in vivo studies are needed to assess ANA pathogenicity in SSc.
A. Representative image of FB and EC following 1-hour incubation with IgG-HC, IgG-ACA+, IgG-ATA+ and poly-ATA (number of patients for each total IgG condition=5). B. Quantification of fluorescently labeled antibodies in FB and EC following 1-hour incubation with distinct IgG serotypes. Ab spot counts were quantified using Imaris in both cytoplasmic and nuclear compartments. A total of 30 cells per condition were analyzed across two to three independent experiments, depending on the serotype. Data are presented as median ± interquartile range (IQR). Statistical significance was assessed using the Kruskal–Wallis test followed by Dunn’s post hoc test; ***p < 0.001. C. Kinetic of fluorescence patterns in FB incubated with poly-ATA for durations ranging from 5 minutes to 6 hours.
A. Three-dimensional FB and EC incubated 1 hour with poly-ATA generated from Z-stack confocal imaging (0.2 μm optical sections). B. FB topoisomerase-I protein co-staining after 1-hour incubation of IgG-ATA+ and FB CENP-B protein co-staining after 1-hour incubation of IgG-ACA+. C. FB and EC after 1 hour with poly-ATA alone, poly-ATA + clathrin endocytosis pathway blockage pretreatment (chlorpromazine) and poly-ATA + caveolin endocytosis pathway blockage pretreatment (genistein).
To cite this abstract in AMA style:
Chepy A, Martel M, Vivier S, Tardivel M, Bongiovanni A, Mistretta M, Secq M, Guilbert L, HACHULLA E, Dubucquoi S, Launay D, Sobanski V. Antinuclear Antibodies from Systemic Sclerosis Patients Enter Cells via a Clathrin Endocytosis Mechanism and Interact with their Intracellular Antigen. [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/antinuclear-antibodies-from-systemic-sclerosis-patients-enter-cells-via-a-clathrin-endocytosis-mechanism-and-interact-with-their-intracellular-antigen/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/antinuclear-antibodies-from-systemic-sclerosis-patients-enter-cells-via-a-clathrin-endocytosis-mechanism-and-interact-with-their-intracellular-antigen/

.jpg)