Session Information
Date: Monday, November 13, 2023
Title: (1155–1182) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: IIM-ILD follows a varied clinical course. Serological profile can help predict clinical phenotype, but impact on ILD prognosis is less clear. This multicentre UK cohort study examines whether serological profile can predict mortality and change in lung function over time.
Methods: Patients with IIM-ILD were identified in 3 NHS trusts from local databases. Adults with ILD meeting IIM diagnostic criteria or Interstitial Pneumonia with Autoimmune Features (IPAF) with Myositis Specific Antibodies (MSA) were included. Baseline characteristics from time of presentation were compared across antibody groups and across survivors/deceased at 2 years. Survival analysis looking at time to death (or lung transplant) for duration of available follow-up was modelled by Cox-Proportional Hazards comparing each antibody individually to all others. Models were adjusted for age, gender, ethnicity, presence of overlap CTD/malignancy, smoking and site. Regression models were also used to observe trends in lung function parameters over time.
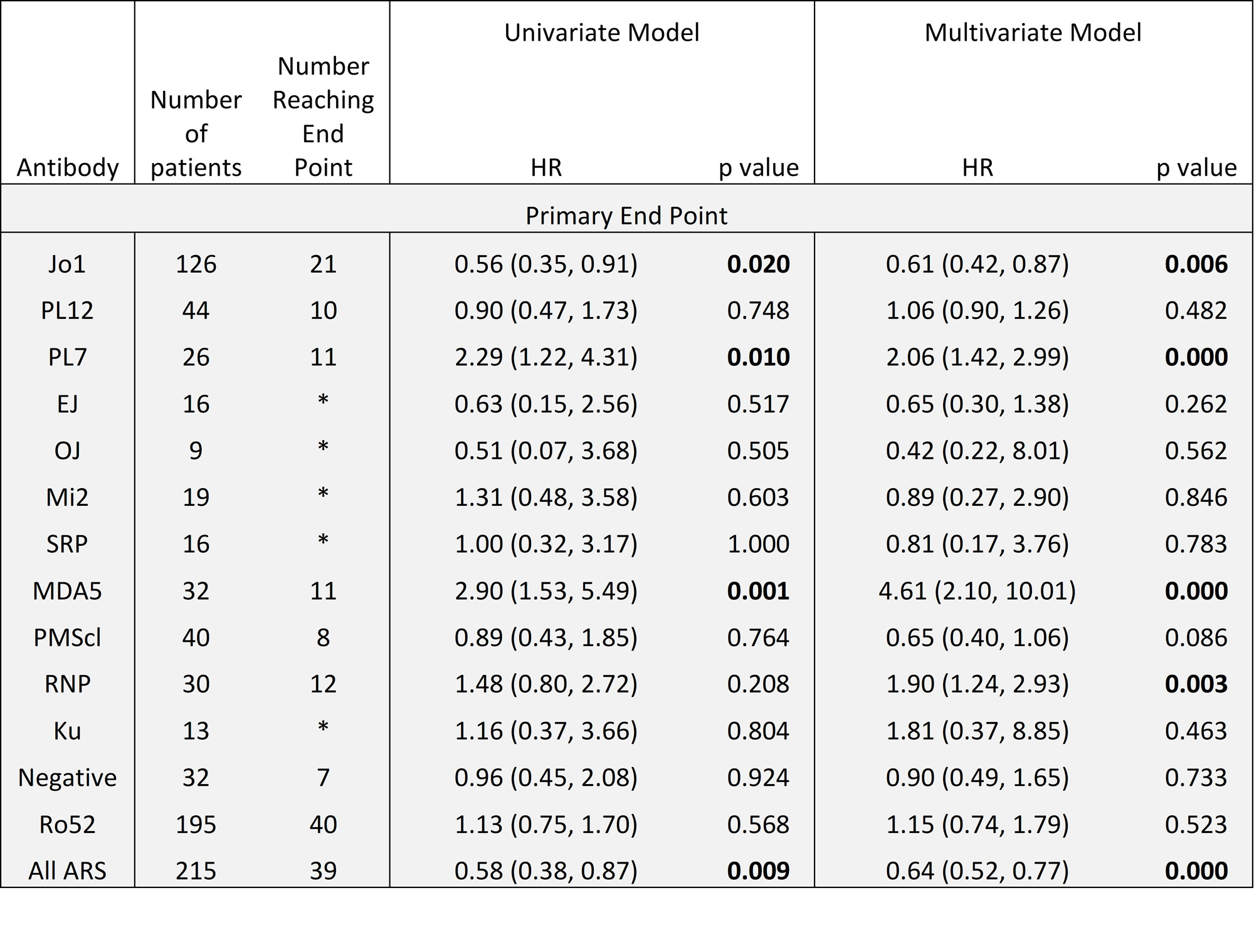
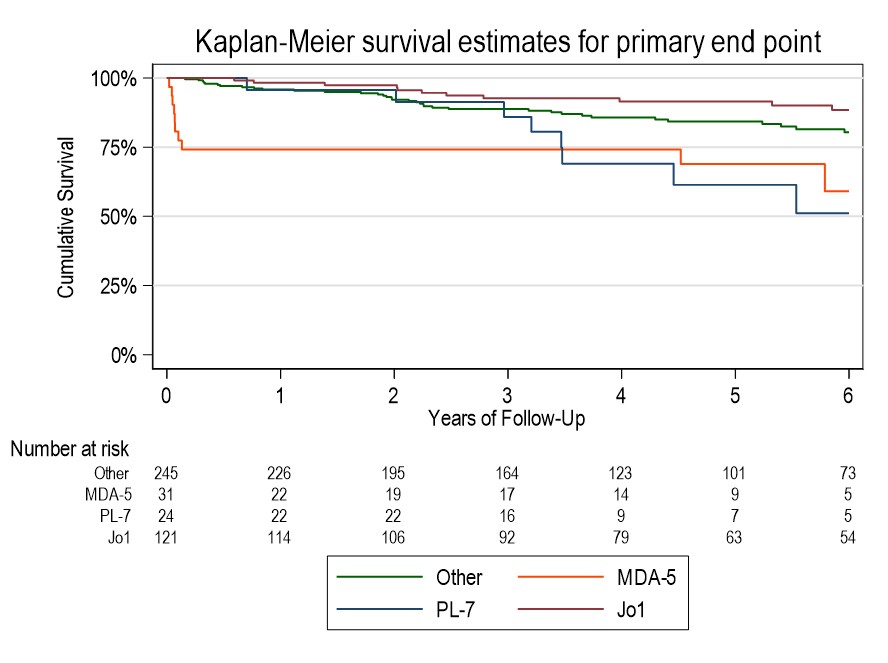
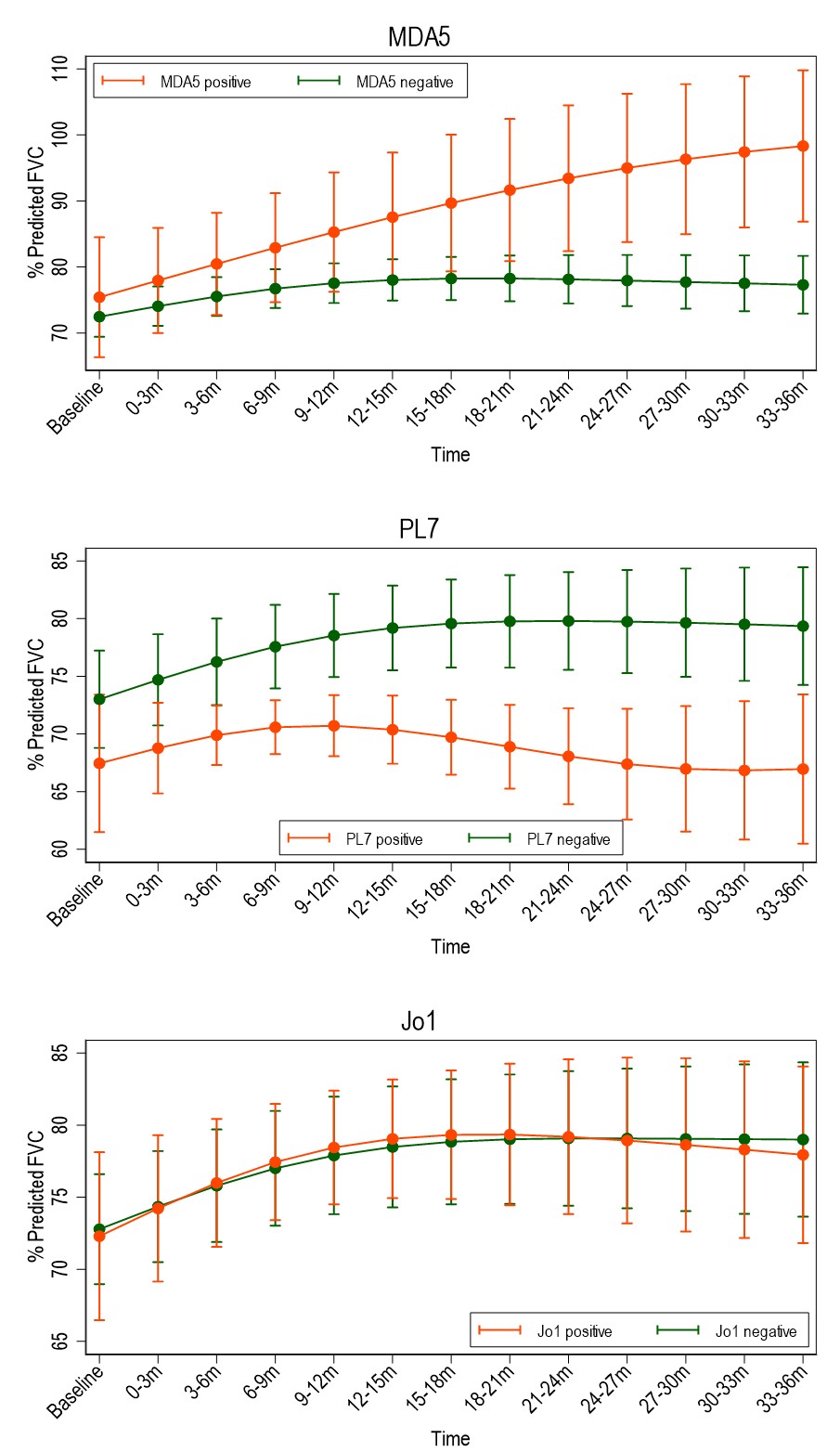
Results: Of 430 included patients, 68% were female, 46% were of White ethnicity. 81% met IIM criteria, 19% were IPAF. Mean follow up duration was 4.3 years. Common antibodies were to Ro52, Jo1, PL12, MDA5 (n=195, 126, 44 & 32 respectively). 10% had evidence of pulmonary hypertension within 1 year of diagnosis, 4% had malignancy within 3 years. Baseline characteristics of survivors vs fatal cases at 2 years showed survivors were younger (51.4 vs 61.7 years), more likely to have never smoked (69% vs 44%), less likely to have been hospitalised at diagnosis (15% vs 52%) and had a lower Charleson Comorbidity Index. Survivors had lower CRP, higher CK & higher baseline FEV1/FVC/TLCO. Imbalance in age, BMI, CK and comorbidity status were seen across antibody groups. MDA5 had the highest adjusted hazard ratio (HR) for mortality of 4.59 (95%CI 2.10-10.01). Kaplan-Meier curve shows high early mortality in this group. Anti-synthetase antibodies (ARS) carried a reduced risk of mortality (HR 0.63), however individually Jo1 had low HR (0.61, 95%CI 0.4-0.87) and PL7 had high HR (2.07, 95%CI 1.44-2.99). RNP showed worse prognosis on adjusted analysis (HR 1.88, 95%CI 1.25-2.84) (Table 1). Regression models suggest that compared to other antibodies, %pred FVC in MDA5 improves over the first 3 years, in PL7 it drops, and in Jo1 it is no different (Figure 1). MDA5 % pred FEV1 also showed improvement, but in Ku it was lower than other antibodies from 21 months. There were no significant differences in % pred TLCO between antibodies.
Conclusion: There is strong evidence that antibody status associates with clinical outcomes, both in terms of progression of lung disease and mortality, suggesting pathogenetic differences. MDA5 predicts a high risk of death early on in disease course, whilst Jo1 associates with lower mortality. PL7 and RNP were additional antibodies associating with higher mortality. Difference between Jo1 and PL7 highlights variation within anti-synthetase syndrome. Paradoxically, if an MDA5 positive person survived the early disease phase, there was evidence that lung function can recover, which may reflect different MDA5 subpopulations.
To cite this abstract in AMA style:
Hannah J, Lawrence A, Martinovic J, Naqvi M, Ali S, Stock C, Owens C, Devaraj A, Pollard L, Agarwal S, Atienza-Mateo B, Patel A, West A, Tinsley K, Robbie H, Chua F, Lams B, Wells A, Norton S, Galloway J, Renzoni E, Gordon P. Antibody Predictors of Prognosis in a Large Multi-centre Cohort of Idiopathic Inflammatory Myopathy Associated Interstitial Lung Disease [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/antibody-predictors-of-prognosis-in-a-large-multi-centre-cohort-of-idiopathic-inflammatory-myopathy-associated-interstitial-lung-disease/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/antibody-predictors-of-prognosis-in-a-large-multi-centre-cohort-of-idiopathic-inflammatory-myopathy-associated-interstitial-lung-disease/