Session Information
Date: Tuesday, October 28, 2025
Title: (2377–2436) Systemic Lupus Erythematosus – Diagnosis, Manifestations, & Outcomes Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Autoantibodies are key diagnostic markers in autoimmune connective tissue diseases (CTD). Conventional testing often requires multiple single-measurand assays, which can be labor-intensive and time-consuming. We evaluated the analytical performance of a multiplex microarray immunoassay (MosaiQ AiPlex® CTDplus; AliveDx, Switzerland) for the simultaneous detection of 15 autoantibodies associated with CTD, used with its fully automated proprietary platform, compared to routine methods in a reference laboratory.
Methods: A cohort of analytically characterized sera (572 reactive and 2,686 non-reactive) were included in the study. Reference methods included a multiplex bead-based immunoassay for most measurands, indirect immunofluorescence assay (IFA) for DFS70, and a chemiluminescence assay for CCP. Samples were tested with the investigational microarray immunoassay that detects IgG autoantibodies to dsDNA, SS-A 60, TRIM21 (SS-A 52), SS-B, Sm, Sm/RNP, U1RNP, Jo-1, Scl-70, CENP B, Chromatin, Ribosomal P, DFS70, RNAP III, and CCP. Performance was assessed using positive (PPA), negative (NPA) and overall percent agreement (OPA). Additionally, Cohen’s kappa coefficient was calculated.
Results: The microarray immunoassay demonstrated substantial agreement with the reference methods (overall Cohen’s kappa: 0.72), with total PPA of 86.6% (95% CI: 83.4, 89.3), total NPA of 92.3% (95% CI: 91.2, 93.3) and total OPA of 91.3%. Individual measurands showed PPA ranging from 59.1% (Sm) to 100% (TRIM21, Jo-1), and NPA ranging from 72.6% (Chromatin) to 100% (Sm, Jo-1, CCP). Notably, high kappa values for almost perfect agreement were observed for SS-A 60 (0.92), TRIM21 (0.85) Sm/RNP (0.97), and CENP B (0.87). Cohen’s kappa values for substantial agreement were observed for dsDNA (0.72), SS-B (0.75) and Sm (0.73); whereas U1RNP (0.49), Scl-70 (0.41) and Ribosomal P (0.43) showed moderated agreement. Finally, kappa values for fair agreement were found for Chromatin (0.33) and CCP (0.34). No samples were available for RNAP III and thus, no comparison was made. Due to limited sample availability, results for Jo-1 (reactive, overall) and CCP (non-reactive, overall) should be interpreted with caution. Detailed results are shown in Table 1.
Conclusion: This fully automated multiplex microarray immunoassay demonstrated overall substantial agreement with established methods for the detection of CTD-associated autoantibodies, with particularly high concordance for key targets such as SS-A 60, Sm/RNP, and CENP B. Its ability to simultaneously detect multiple autoantibodies in a single, automated run may streamline the serologic evaluation and support more efficient clinical workflows in autoimmune diagnostics.
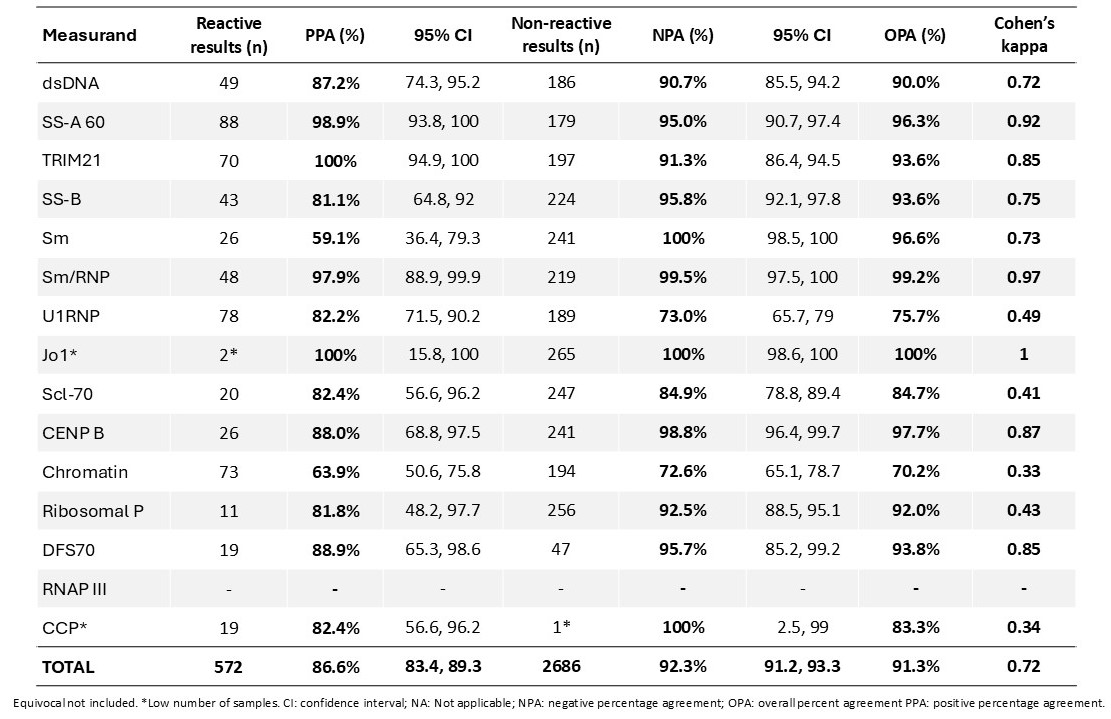 Table 1. Agreement of the Microarray Immunoassay with Comparators
Table 1. Agreement of the Microarray Immunoassay with Comparators
To cite this abstract in AMA style:
Nardella G, Gomez G, Fischer C, Monat C. Analytical Performance of a Fully Automated Multiplexed Microarray Immunoassay for the Simultaneous Detection of Fifteen Autoantibodies Associated with Connective Tissue Diseases in a Reference Laboratory in Southern France [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/analytical-performance-of-a-fully-automated-multiplexed-microarray-immunoassay-for-the-simultaneous-detection-of-fifteen-autoantibodies-associated-with-connective-tissue-diseases-in-a-reference-labora/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/analytical-performance-of-a-fully-automated-multiplexed-microarray-immunoassay-for-the-simultaneous-detection-of-fifteen-autoantibodies-associated-with-connective-tissue-diseases-in-a-reference-labora/
