Session Information
Date: Saturday, November 16, 2024
Title: Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: To investigate the effect of colchicine prophylaxis on gout remission when commencing urate lowering therapy (ULT), and illness perceptions of people in remission, using two definitions of gout remission.
Methods: Data from a 12-month double-blind placebo-controlled trial of 200 people with gout commencing allopurinol were analyzed1. Participants were randomly assigned to prophylaxis with 0.5mg daily colchicine or placebo for six months, followed by six months of additional follow-up. Gout remission was assessed using the 2016 preliminary definition2 or simplified definition without patient reported outcomes. Binary logistic regression was used to compare intervention groups. Illness perceptions were assessed using a gout-specific brief illness perception questionnaire (BIPQ).
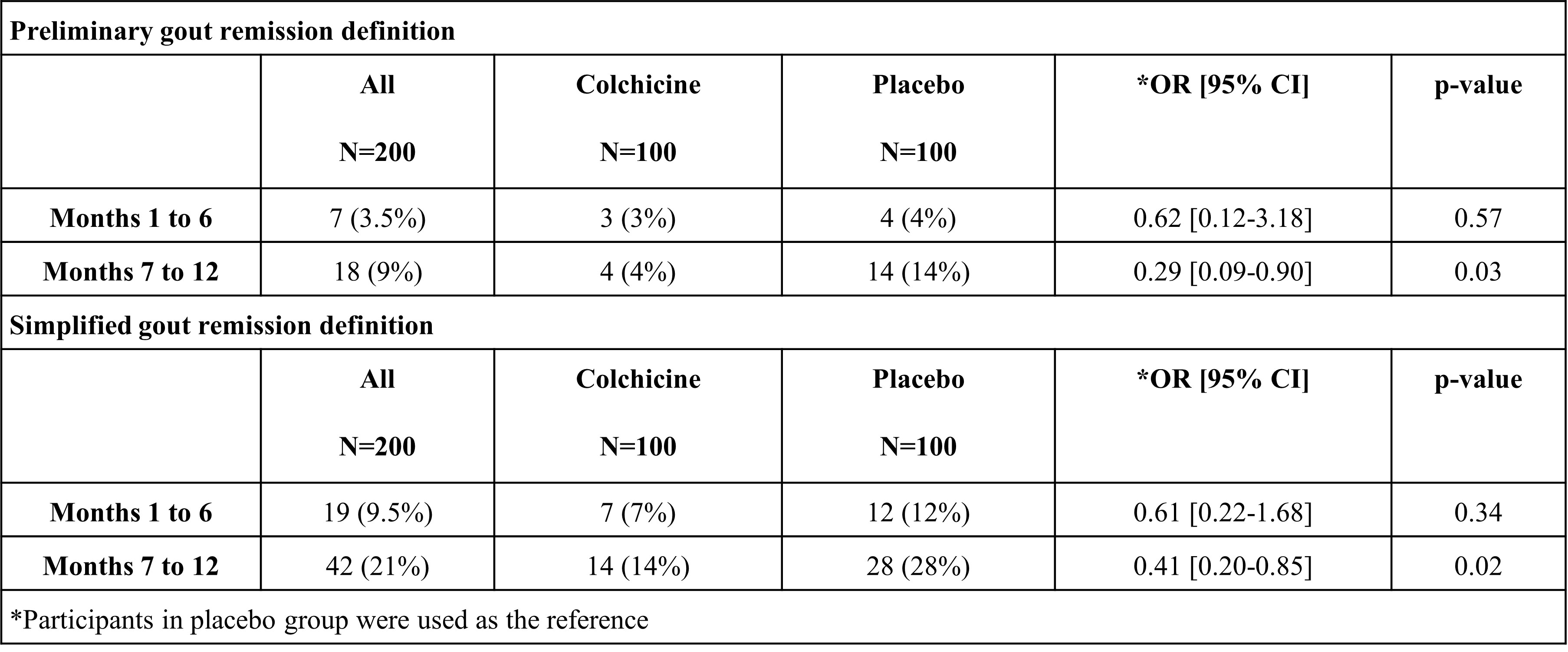
Results: In the first six months, few participants were in remission according to either the 2016 preliminary definition (3% for colchicine and 4% for placebo) or the simplified definition (7% for colchicine and 12% for placebo) (Table 1). In the second six months, after study drug (colchicine or placebo) discontinuation, fewer participants in the colchicine group than in the placebo group were in remission according to the 2016 preliminary definition (4% vs. 14%, p=0.03), and the simplified definition (14% vs 28%, p=0.02) (Table 1). Participants fulfilling remission using either definition had more favorable perceptions about their gout symptoms and illness concerns, as well as consequences, when using the simplified definition.
Conclusion: Using either definition, six months of colchicine prophylaxis when initiating ULT does not provide an advantage in the fulfilment of gout remission. People fulfilling either definition report fewer symptoms, lower concern about their gout, and when using the simplified definition, are less affected by gout.
1. Stamp et al, Ann Rheum Dis 2023; 82:1626-1634.
2. de Lautour et al, Arthritis Care Res 2016;68:667-72.
To cite this abstract in AMA style:
Tabi-Amponsah D, Stamp L, Horne A, Drake J, Stewart S, Gamble G, Petrie K, Dalbeth N. Analysis of Gout Remission Definitions in a Randomised Controlled Trial of Colchicine Prophylaxis for People with Gout Initiating Allopurinol [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/analysis-of-gout-remission-definitions-in-a-randomised-controlled-trial-of-colchicine-prophylaxis-for-people-with-gout-initiating-allopurinol/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/analysis-of-gout-remission-definitions-in-a-randomised-controlled-trial-of-colchicine-prophylaxis-for-people-with-gout-initiating-allopurinol/