Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: A variant of HLA-DR confers the strongest genetic risk for rheumatoid arthritis (RA) suggesting that DRhi cells are important in RA. We previously found that RA blood contains ‘unorthodox’ DRhi immune cells (non-lymphoid cells that do not conform to bona fide definitions of monocytesnor dendritic cells).The purpose of the study was to determine whether DRhi immune cells expressing granulocyte associated molecules (CD15, CCR3)contribute to these alterations of the DRhi pool in RA.
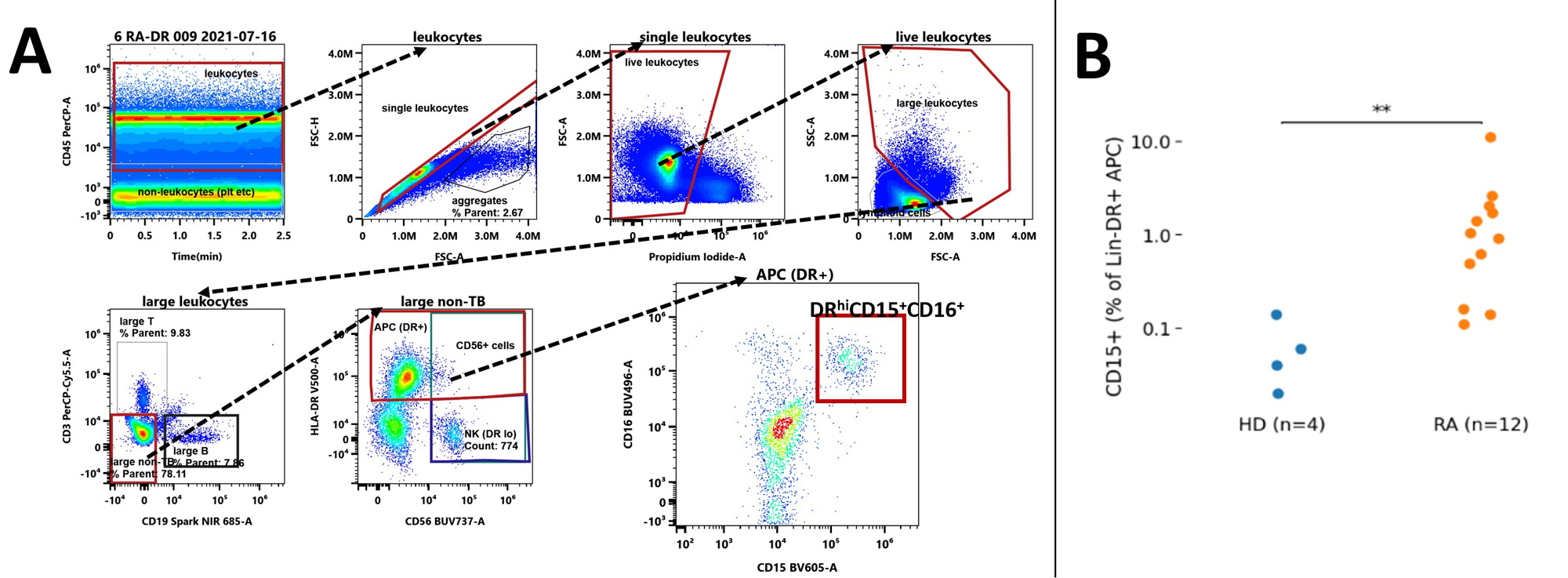
Methods: We studied RA patients (n=12) satisfying the 2010 ACR classification criteria and matched healthy donors by flow cytometry. PBMC and other low-density cells were isolated from blood by Ficoll density gradient centrifugation. To gate non-lymphoid DRhi cells we excluded lymphocytes and non-viable cells based onforward and side scatter, CD3/CD19 dump and viability gates (Fig. 1A). We quantified the contribution of CD15+ cells to the non-lymphoid DRhi pool and their expression (MFI) of co-stimulatory and co-inhibitory molecules. We used t-SNE on index patients (debilitating polyarticular synovitis)to clarify the higher-dimensional structure of the CD15positive DRhi subpopulation followed by bi-axial gating to validate anyt-SNE guided observation and quantify distinctive features of DRhiCD15+. Kruskal-Wallis testing with a threshold of p< 0.05 was performed to assess for significant differences.
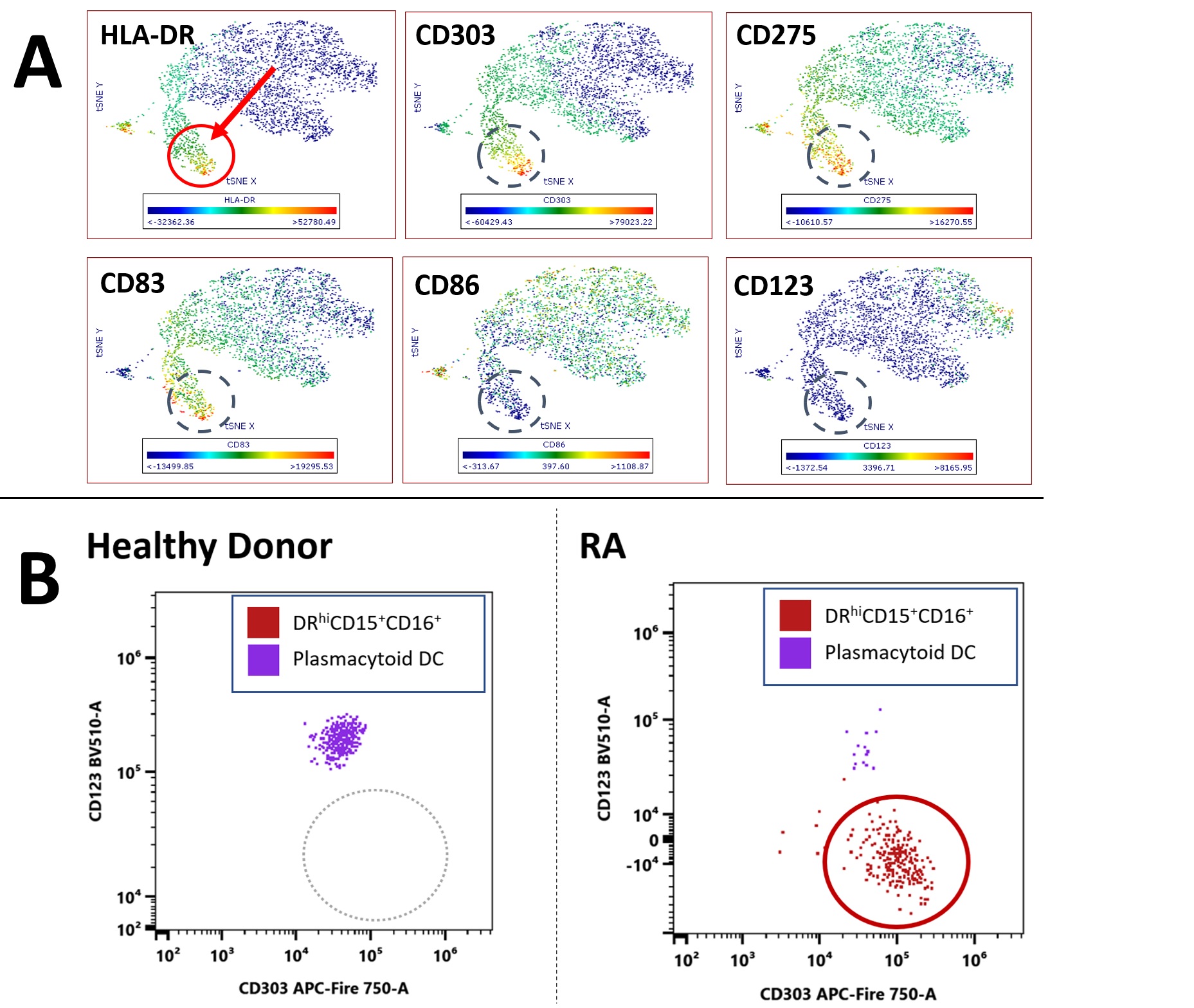
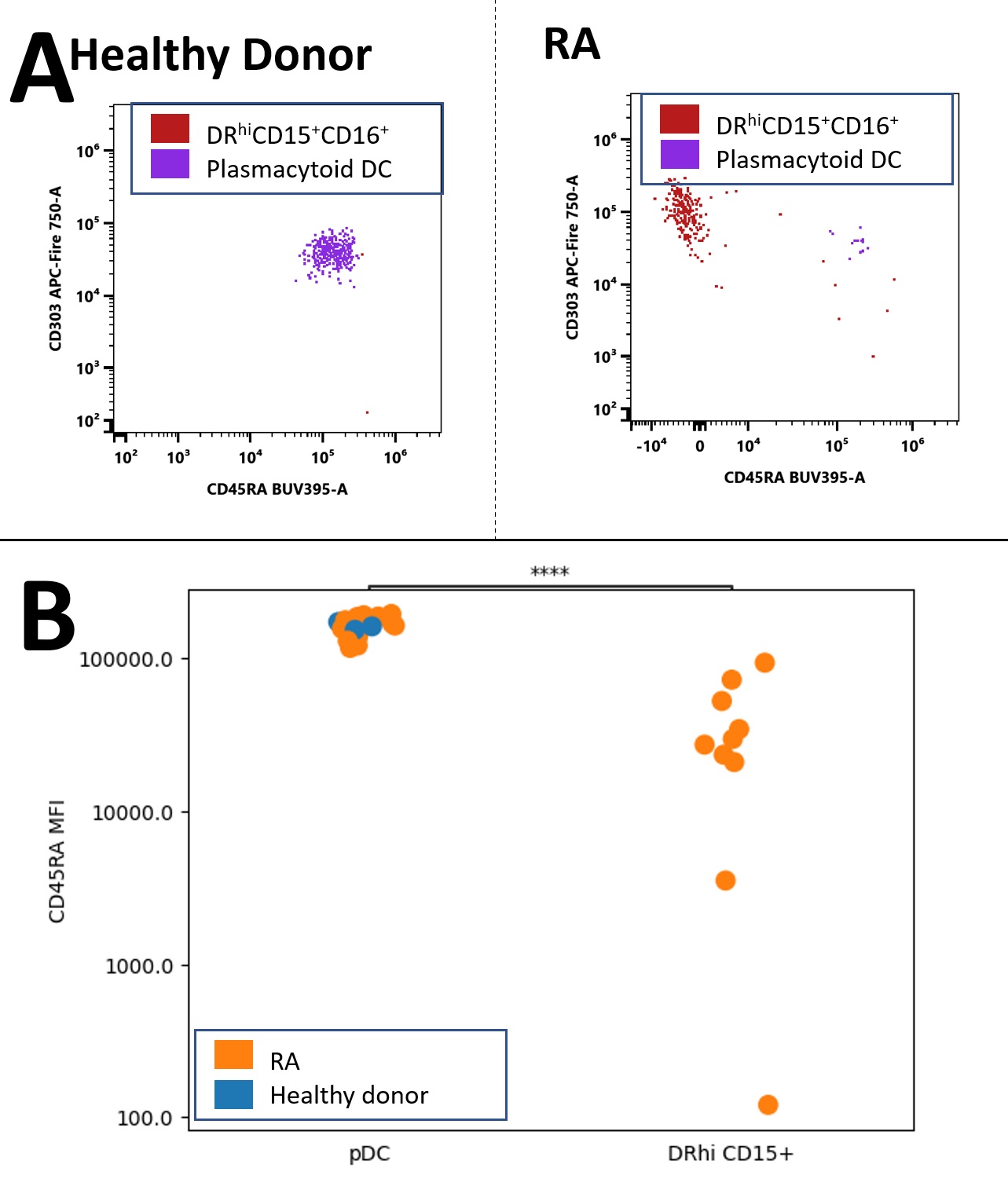
Results: In RA we found a non-lymphoid DRhi CD15+ population that was virtually absent in healthy donors (HD)(RA 0.98% vs. HD 0.05% of non-lymphoid DRhi; p<0.01, Fig. 1B, 2B); these cells have near uniform CD16 co-expression (Fig 1A, gated in last plot).DRhi CD15+ showed—as expected from granulocytic cells — high side scatter but differed from granulocytes by a striking co-expression of plasma cytoid (pDC) marker CD303 (Fig. 2A, B; circled);CD123, highly expressed by pDC, was not expressed. Given the shared features with both granulocytes and pDC we refer to this population as DRhi ‘Hybrid’ cells. DRhi Hybrids formed a separate cluster in RA which, along with CD303, co-expressed CD83 and CD275 (ICOS-L) (Fig. 2A, circled population).Lack of CD45RAseparatedCD303+DRhi Hybrids from their apparent bone fide CD303+ CD45RAint pDC counterparts (Fig. 3A and B, p< 0.001), CD45RA expression of RA pDC and HD pDC did not differ (Fig 3B, left). CCR3 expression within non-lymphoid DRhi was negligible (not shown).
Conclusion: RA blood contains DRhiCD15+ cells, contributing to the non-lymphoid DRhi pool in RA. Because these low-density cells share features with both granulocytes and pDCs we refer to them as DRhi Hybrids; their expression of CD303 challenges the notion that this molecule is specific for plasmacytoid DC. Co-expression of CD83, CD275 suggests an inflammatory potential whereas their lack of CD45RA (as opposed to CD303+pDCs) is reminiscent of the altered CD45RA expression we found previously in a DC2-like DRhi subset. Joint expression of CD303 and DR suggests that DRhi Hybrids maybe functionally involved in the capture and ultimate presentation of RA self-peptides to T lymphocytes. These apparently new DRhi Hybrids and other unorthodox DRhi populations are potential treatment targets in RA.
To cite this abstract in AMA style:
Geier C, Qudsi H, BenGabr J, Winchester R, Perl A. An Unorthodox HLA-DRhiCD15+ ‘Hybrid’ Population in Rheumatoid Arthritis Characterized Using Spectral Cytometry [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/an-unorthodox-hla-drhicd15-hybrid-population-in-rheumatoid-arthritis-characterized-using-spectral-cytometry/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/an-unorthodox-hla-drhicd15-hybrid-population-in-rheumatoid-arthritis-characterized-using-spectral-cytometry/