Session Information
Session Type: Abstract Session
Session Time: 5:00PM-5:50PM
Background/Purpose: Thy1 (CD90) is a glycosylated, glycophosphatidylinositol (GPI)-anchored membrane protein noted to be expressed on many cells including T lymphocytes, stem cells, osteoblasts and fibroblasts. Thy1 is also abundantly expressed by mesenchymal stem cells (MSCs) and we previously demonstrated that it is required for osteoblast differentiation and bone homeostasis. The goal of this study was to examine if knockout of the membrane protein Thy1 (CD90), is associated with increased pathologic bone resorption in a murine model of inflammatory arthritis.
Methods: We performed real-time PCR (RT-PCR) to compare Thy1 mRNA expression levels in mouse bone marrow cells and monocyte-derived dendritic cells (mDCs) of TNF overexpressing (TNF-Tg) and wild type (WT) mice and murine embryonic fibroblasts (MEFs) from (WT) mice. Subsequently, we also investigated the impact of the Thy1 deletion in TNF-Tg (Tg3647) mice, a murine model of inflammatory arthritis. To study the effect of Thy1 depletion on bone erosion, we analyzed bone loss by performing micro-computed tomography (micro-CT) analysis of the long bones, and ankle joints of Thy1-/-TNF-Tg, TNF-Tg, Thy1-/- and WT mice.
Results: In vitro assays revealed a significant decrease in Thy1 expression (40% – 65%) in primary bone marrow cells and mDCs of the TNF-Tg mice compared to the WT mice (Figure 1). Thy 1 mRNA levels decreased by more than 25% in murine embryonic fibroblasts following exposure to TNF for 24 hours. Most importantly, micro-CT analysis of the long bone and ankle joints revealed a significant increase in bone loss in the Thy1-/-TNF-Tg mice, compared to the TNF-Tg or Thy1-/- mice (Figure 2). In particular, a significant reduction in bone parameters were observed in Thy1-/- TNF-Tg mice compared to the TNF-Tg, Thy1-/- or WT mice. Significant differences were noted in BV/TV (0.003 ± 0.02 in Thy1-/-TNF-Tg vs 0.024± 0.01 in TNF-Tg, p < 0.01, for tibia), trabecular number (1.57 ± 0.2 in Thy1-/-TNF-Tg vs 2.4 ± 0.1 in TNF-Tg, p < 0.005, for tibia), and trabecular thickness (0.026 ± 0.002 in Thy1-/-TNF-Tg vs 0.032 ± 0.01 in TNF-Tg, p < 0.01, for tibia). Similar bone loss was not noted in WT or Thy1 KO mice.
Conclusion: We find Thy1 expression significantly decreased in MSCs and myeloid cells following exposure to TNF. Absence of Thy1 was associated with enhanced pathologic bone resorption in murine model of arthritis. Thus, TNF-mediated downregulation of Thy 1 may serve as an important mechanistic link between inflammation and progressive bone loss in inflammatory arthritis.
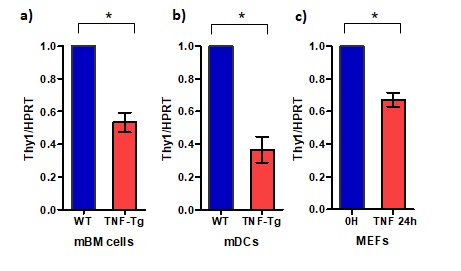 Figure 1: TNF exposure downregulates Thy1 expression in RA SFs, MEFs, BM cells, and mDCs a) Thy1 expression level in SFs from RA patients incubated with or without TNF for 12 hours (microarray data: GSE13837, fold change, n=3); b) MEFs were incubated with or without 5 ng/ml human TNF for 24 hours and thereafter Thy1 expression levels were compared using qPCR (n=3); c) Thy1 expression levels in freshly harvested bone marrow cells from 6 months old male WT and TNF-Tg mice (n=4); d) Thy1 mRNA expression levels in the monocyte-derived DCs of 4-month-old female WT and TNF-Tg mice (n=4). For qPCR data, HPRT1 was used as the housekeeping gene.
Figure 1: TNF exposure downregulates Thy1 expression in RA SFs, MEFs, BM cells, and mDCs a) Thy1 expression level in SFs from RA patients incubated with or without TNF for 12 hours (microarray data: GSE13837, fold change, n=3); b) MEFs were incubated with or without 5 ng/ml human TNF for 24 hours and thereafter Thy1 expression levels were compared using qPCR (n=3); c) Thy1 expression levels in freshly harvested bone marrow cells from 6 months old male WT and TNF-Tg mice (n=4); d) Thy1 mRNA expression levels in the monocyte-derived DCs of 4-month-old female WT and TNF-Tg mice (n=4). For qPCR data, HPRT1 was used as the housekeeping gene.
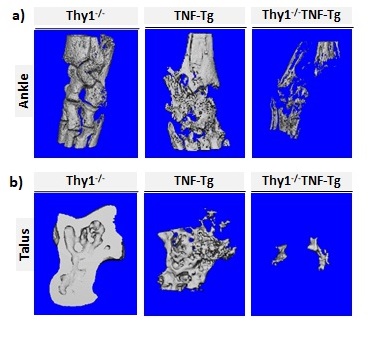 Figure 2. Increased bone loss in Thy1 KO TNF-Tg mice. Long bones from normal diet fed 8 months old male Thy1-/-, TNF-Tg, and Thy1-/-TNF-Tg mice were analyzed with micro-CT to compare changes in bone architecture and structural parameters. Representative micro-CT images of the ankle (a), and talus (b) presented.
Figure 2. Increased bone loss in Thy1 KO TNF-Tg mice. Long bones from normal diet fed 8 months old male Thy1-/-, TNF-Tg, and Thy1-/-TNF-Tg mice were analyzed with micro-CT to compare changes in bone architecture and structural parameters. Representative micro-CT images of the ankle (a), and talus (b) presented.
To cite this abstract in AMA style:
Paine A, Garcia-Hernandez M, Nuzzo M, Duemmel S, Korman B, Ritchlin C. Absence of Thy1 Associated with Severe Bone Loss in the TNF-transgenic (TNF-Tg) Mice Arthritis Model [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/absence-of-thy1-associated-with-severe-bone-loss-in-the-tnf-transgenic-tnf-tg-mice-arthritis-model/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/absence-of-thy1-associated-with-severe-bone-loss-in-the-tnf-transgenic-tnf-tg-mice-arthritis-model/
