Session Information
Date: Sunday, October 26, 2025
Title: (0067–0097) Rheumatoid Arthritis – Etiology and Pathogenesis Poster
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Fibroblast-like synoviocytes (FLS) play a central role in cartilage destruction and cytokine production in rheumatoid arthritis (RA). Neddylation, a post-translational modification involving NEDD8 conjugation, is a key regulator of inflammatory signaling. NUB1, a negative regulator of neddylation, is dysregulated in FLS with defective induction after cytokine stimulation that can lead to increased NF-kB activation. Previous studies have shown that this defect is associated with differential histone modification at the NUB1 promoter between RA and osteoarthritis (OA). However, the molecular mechanism of insufficient NUB1 induction in RA remains unclear.
Methods: FLS were isolated from RA and OA synovial tissues and used from passages 4–8. To evaluate mRNA half-life, cells were stimulated with IL-1β (2 ng/mL) for 6 hours, followed by treatment with actinomycin D (10 μg/mL) to interrupt transcription. SNHG12, a long non-coding RNA that binds to NUB1 was depleted with SNHG12 siRNA (knockdown >85). Gene expression was quantified by RT-qPCR after IL-1 stimulation in the presence of EPZ6438 (a histone methylation inhibitor) or MS-275 (an HDAC1/HDAC3 inhibitor).
Results: We initially confirmed that NUB1 induction was deficient in RA compared with OA FLS after IL-1 stimulation (Fig. 1). Actinomycin D treatment revealed no significant difference in NUB1 mRNA half-life between RA and OA FLS that might account for this difference, with the half-life for RA=11.3h and for OA=10.7h. In addition, SNHG12 deficiency did not affect NUB1 gene expression or induction in either RA and OA FLS. These findings suggest that the differential expression of NUB1 between RA and OA is likely due to transcriptional differences rather than post-transcriptional regulation. Because NUB1 regulatory regions have distinct epigenetic marks in RA compared to OA, we evaluated this possibility by treated the cells with epigenetic modulators and then stimulated FLS with IL-1 (Fig. 2). IL-1 stimulation resulted in significantly higher induction of NUB1 mRNA in OA FLS compared to RA FLS (RA: 1.6-fold, OA: 3.0-fold; p = 0.01). EPZ treatment partially reversed this difference (see Fig. 2). MS-275 completely reversed differential induction of NUB1 in OA compared with RA. In addition, 2 week treatment with a DNA methylation inhibitor 5-azacytidine did not alter differential induction of NUB1 in RA compared with OA.
Conclusion: The mechanism of reduced induction of NUB1 in RA FLS is related to differential histone marks in the promoter, most notably histone methylation and acetylation. Because low levels of NUB1 are associated with increased NF-kB activation and cytokine production, this could contribute to increased neddylation and cytokine production in RA FLS. Therapeutic approaches that modify the epigenetic landscape in RA could potentially suppress the inflammatory milieu.
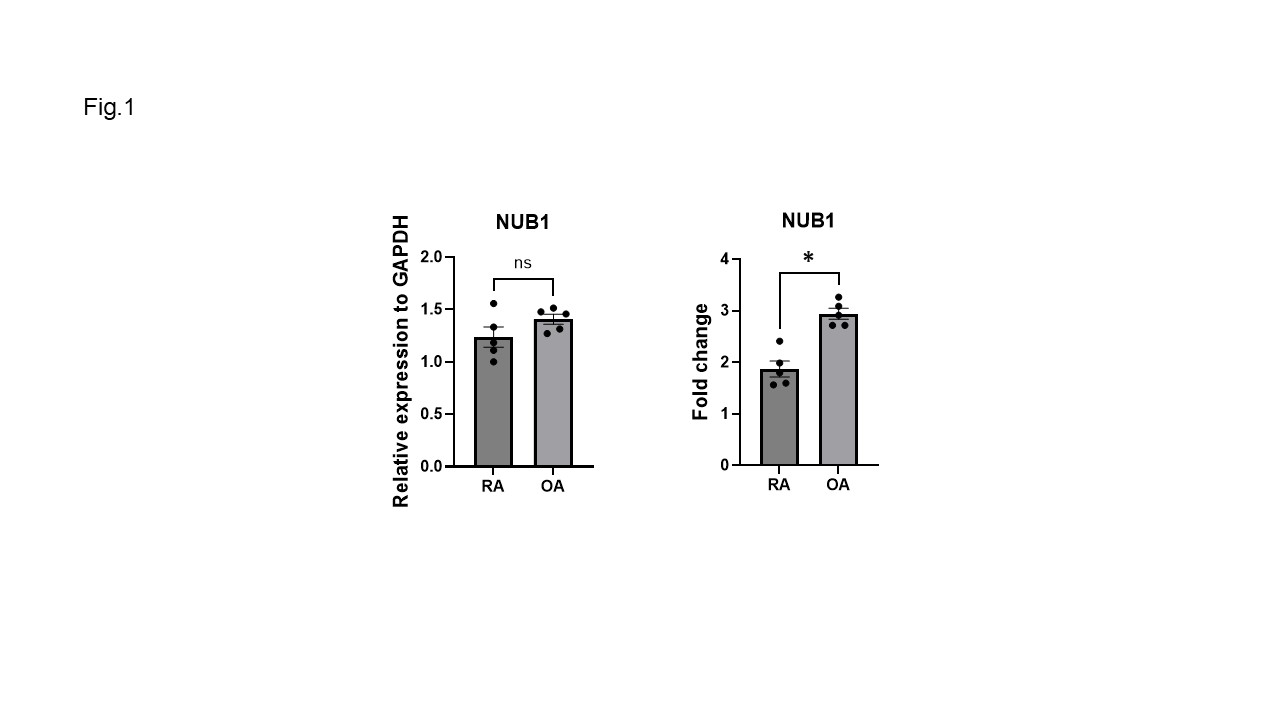 (Fig.1) Reduced induction of NUB1 by IL-1 in RA FLS compared to OA FLS. Basal and IL-1-induced expression of NUB1 in RA and OA FLS is shown. Left: Basal NUB1 mRNA expression in RA and OA FLS, normalized to GAPDH. Right: Fold change in NUB1 expression following IL-1 stimulation (2ng/mL, 6 h), relative to unstimulated (DMSO-treated) cells (RA and OA, n=5 each). Bars represent mean values; individual dots represent biological replicates. Error bars indicate SEM. *; p < 0.05, ns; not significant.
(Fig.1) Reduced induction of NUB1 by IL-1 in RA FLS compared to OA FLS. Basal and IL-1-induced expression of NUB1 in RA and OA FLS is shown. Left: Basal NUB1 mRNA expression in RA and OA FLS, normalized to GAPDH. Right: Fold change in NUB1 expression following IL-1 stimulation (2ng/mL, 6 h), relative to unstimulated (DMSO-treated) cells (RA and OA, n=5 each). Bars represent mean values; individual dots represent biological replicates. Error bars indicate SEM. *; p < 0.05, ns; not significant.
.jpg) (Fig.2) Reversal of NUB1 differential induction between RA and OA FLS by epigenetic modifiers EPZ6438 (histone methylation inhibitor) and MS-275 (HDAC1/HDAC3 inhibitor). RA and OA FLS were stimulated with IL-1β (2 ng/mL) after pretreatment with epigenetic inhibitors. Fold change was calculated as the ratio of NUB1 expression in IL-1–stimulated cells to that in the corresponding unstimulated control. For the IL-1/DMSO condition, fold change reflects expression relative to DMSO alone. For inhibitor-treated conditions, fold change was calculated relative to cells treated with the same inhibitor without IL-1 stimulation. Circles and squares represent mean fold change for OA and RA FLS, respectively (n = 5 each). *; p < 0.05
(Fig.2) Reversal of NUB1 differential induction between RA and OA FLS by epigenetic modifiers EPZ6438 (histone methylation inhibitor) and MS-275 (HDAC1/HDAC3 inhibitor). RA and OA FLS were stimulated with IL-1β (2 ng/mL) after pretreatment with epigenetic inhibitors. Fold change was calculated as the ratio of NUB1 expression in IL-1–stimulated cells to that in the corresponding unstimulated control. For the IL-1/DMSO condition, fold change reflects expression relative to DMSO alone. For inhibitor-treated conditions, fold change was calculated relative to cells treated with the same inhibitor without IL-1 stimulation. Circles and squares represent mean fold change for OA and RA FLS, respectively (n = 5 each). *; p < 0.05
To cite this abstract in AMA style:
Ono Y, Machado C, Choi E, Wang W, Boyle D, Firestein G. Aberrant histone marks increase the inflammatory phenotype of rheumatoid arthritis fibroblast-like synoviocytes (RA FLS) by suppressing NUB1 induction [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/aberrant-histone-marks-increase-the-inflammatory-phenotype-of-rheumatoid-arthritis-fibroblast-like-synoviocytes-ra-fls-by-suppressing-nub1-induction/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/aberrant-histone-marks-increase-the-inflammatory-phenotype-of-rheumatoid-arthritis-fibroblast-like-synoviocytes-ra-fls-by-suppressing-nub1-induction/
