Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Patients have no comprehensive, curated resource on steroid use to facilitate effective collaboration in their own care. Consequently, truly shared decision making around steroid treatment is challenging. The SAM program – short for “Steroids and Me” – is a collaboration among academic physicians, patients, patient advocacy groups, and industry leaders to develop a patient-focused program to help patients optimize their steroid use. We report on SAM’s two years of development and first five months of use.
Methods: SAM’s development plan was divided into three phases. Phase 1 (Resource Review) was a survey of the medical literature and internet for current, scientifically rigorous, patient-focused content on steroid use in clinical care. Phase 2 (Needs Assessment) consisted of two international roundtable meetings of patient advocacy group leaders and industry representatives (total participants, n=36); separate meetings with 6 patient advocacy groups; 3 patient focus groups; and meetings with industry representatives from 5 companies. Finally, Phase 3 (SAM Build) consisted of building the web-based platform accessible by phone, tablet or computer. In this phase, a 34-member patient group was engaged in one-on-one meetings for user testing and patient journey validation.
Results: SAM is protected by stringent privacy policies and fulfills requirements of both HIPAA/GDPR and state-specific data privacy laws. The development program launched at ACR Convergence 2022 and was researched and built over a two-year period. In Phase 1 (Resource Review), audits were performed on 54 patient advocacy and patient-facing industry websites. Although the medical literature contains abundant documentation of the damage caused by steroid use, there is no dedicated instruction for patients about how to mitigate these side-effects: the Resource Review identified not a single source of curated, comprehensive, patient-focused material on steroid toxicity. In Phase 2 (Needs Assessment), patient advocacy groups representing lupus, vasculitis, pemphigus, myasthenia gravis, sarcoidosis, and IgG4-related disease participated. Discussions with individual patients and groups of patients identified major unmet needs that were amplified by patient advocacy group leaders and industry (Table 1).
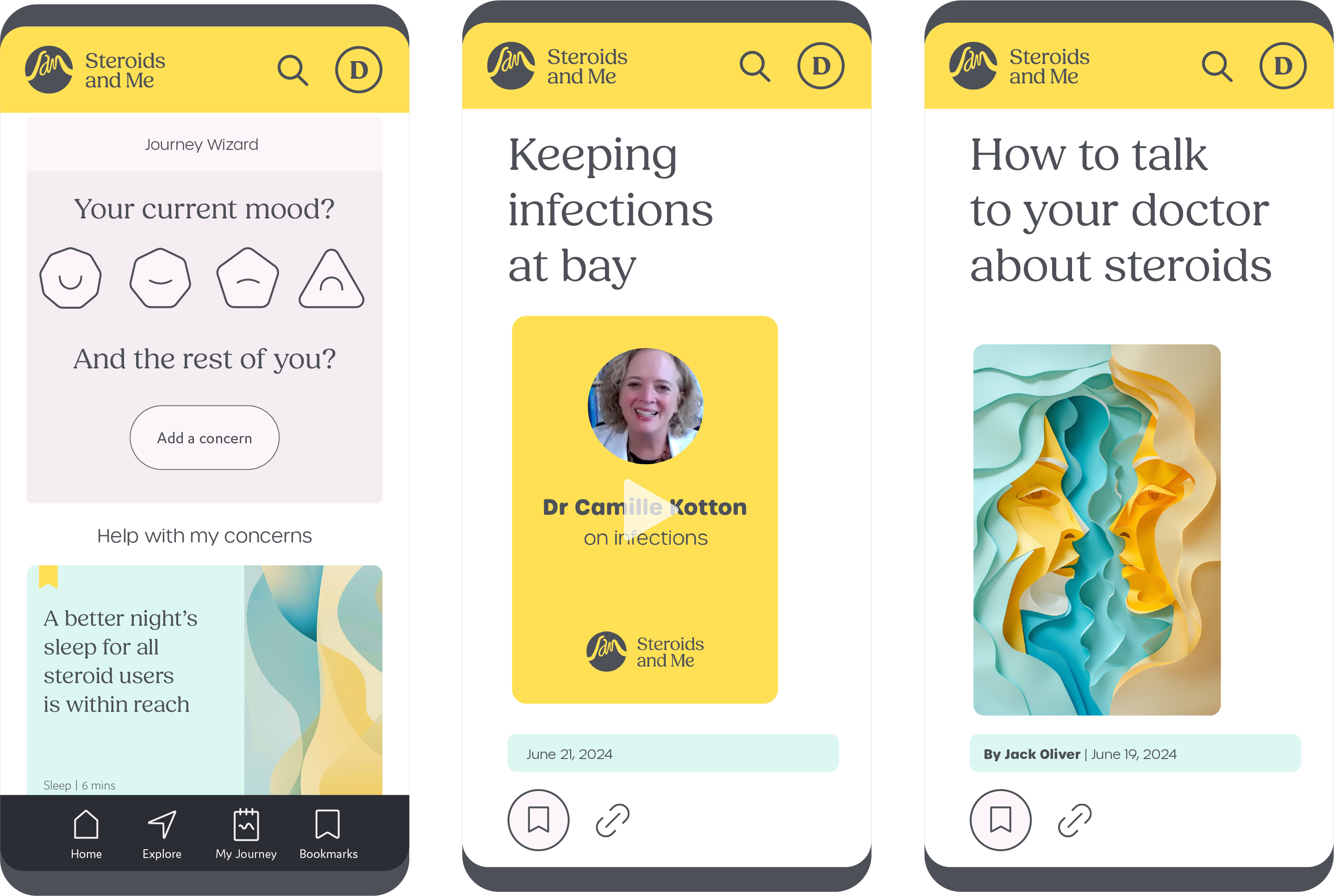
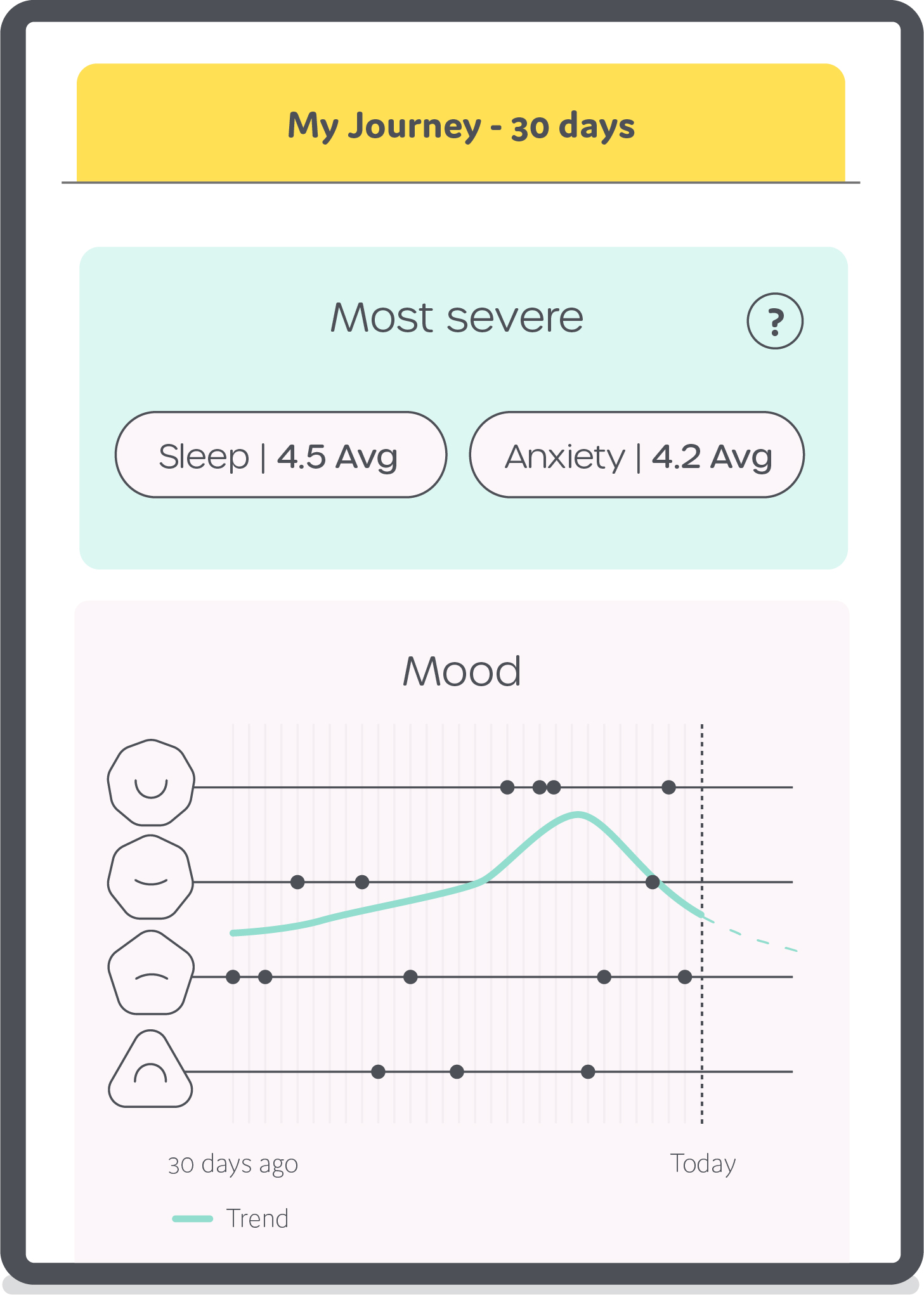
All SAM visitors can access educational content, including written material on dozens of known steroid side-effects; tips about the prevention and management of steroid toxicities; and videos created by physician experts to supplement those educational tools (Figure 1). All content is original, developed and recorded by internationally-recognized clinicians. SAM also provides tools, including mood and side effect tracking, that prepare patients for shared decision-making conversations with their providers. SAM collects and stores longitudinal patient-reported data, and displays a 30-day look back to summarize side-effects in anticipation of follow up clinic visits (Figure 2).
Conclusion: Steroids and Me (SAM) is a unique patient-focused resource that engages, educates, and equips users to understand and reduce steroid-toxicity, facilitating conversations that foster shared decision making around steroid use.
To cite this abstract in AMA style:
Stone J, Petri M, Gelfand J, Kotton C, McDowell J, Papaliodis G, Marinaro m, Wilkinson M, Lentfert W, Stone M. A Patient-Focused Program for Using Steroids Wisely [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-patient-focused-program-for-using-steroids-wisely/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-patient-focused-program-for-using-steroids-wisely/