Session Information
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Rheumatoid arthritis (RA) is characterized by autoantibodies against post-translationally modified proteins (AMPA) such as citrullinated, carbamylated and acetylated proteins. Importantly, these antibodies are highly multireactive, as they often recognize more than one of these post-translational modifications. Despite extensive research, the antigens inducing the breach of tolerance remain unknown, although microbial antigens are often suspected. Various bacteria are known to be capable of acetylation, therefore, it is intriguing to know which mechanisms underlie the breach of tolerance towards acetylated proteins and development of AMPA. The aim of this project was to investigate whether acetylated proteins of bacterial origin (1) are recognized by human derived AMPA and AMPA-expressing B cells; and (2) can induce AMPA development when used to immunize mice.
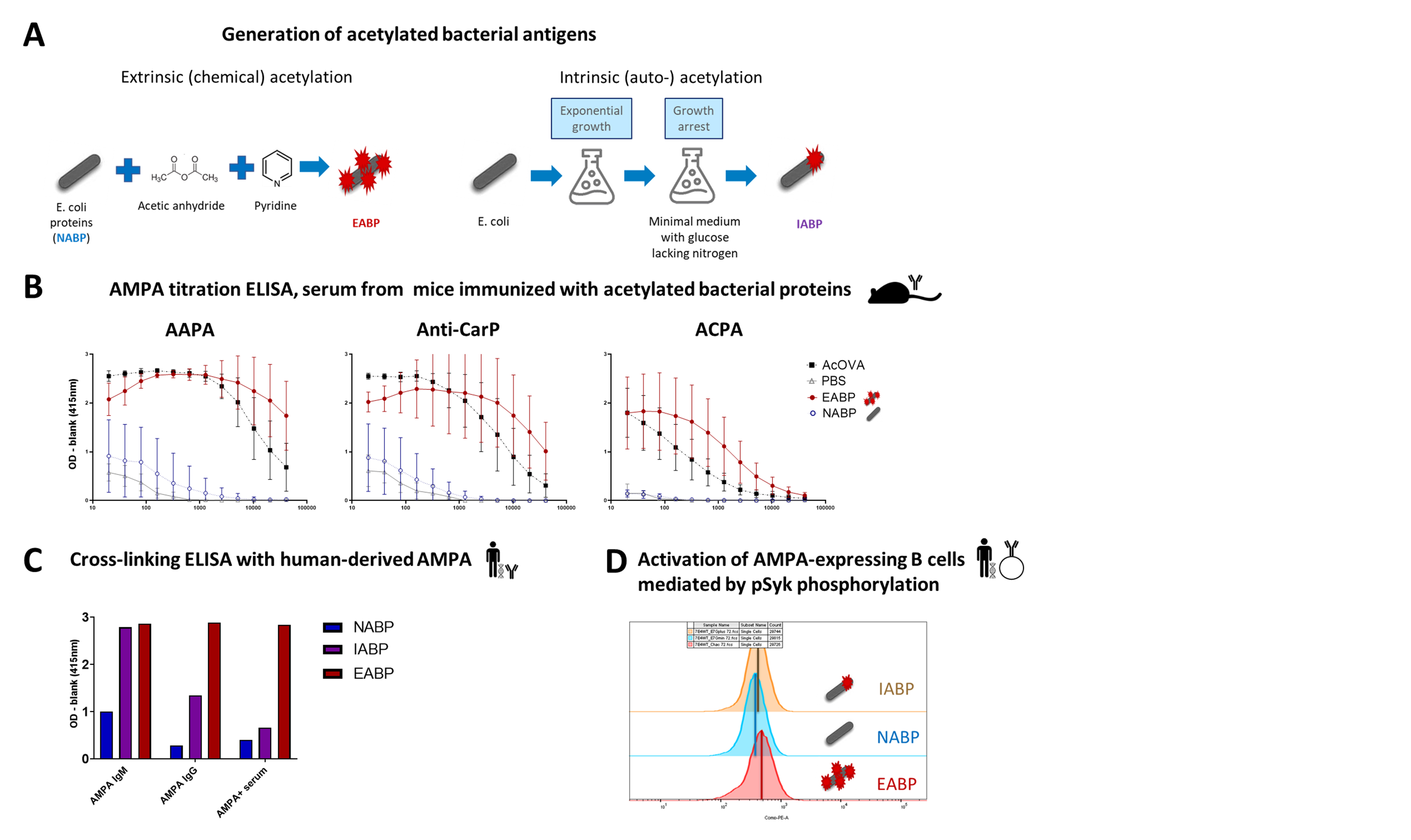
Methods: Acetylated E. coli proteins were acquired with two separate methods (Figure A): by culturing E. coli in a condition promoting auto-acetylation (intrinsically acetylated bacterial proteins, IABP), or by directly acetylating lysate-derived proteins via a chemical reaction (extrinsically acetylated BP, EABP). Acetylated ovalbumin (AcOVA) served as positive control for AMPA induction in mice, while non-acetylated BP (NABP) and phosphate buffer saline (PBS) served as negative control. Mice were immunized with these proteins and the resulting antibody response was studied by ELISA. Furthermore, EABP/IABP/NABP were investigated for recognition by human-derived AMPA with ELISA and AMPA-expressing B cells with spleen tyrosine kinase (Syk) phosphorylation assay; acetylated human fibrinogen and native fibrinogen served as positive and negative control.
Results: Intrinsic acetylation resulted in partial, while extrinsic/chemical acetylation resulted in complete acetylation of E. coli proteins. Repetitive immunization of mice with EABP resulted in an AMPA response recognizing acetylated, carbamylated and citrullinated proteins, which was not observed in mice immunized with NABP or IABP (Figure B). Human-derived AMPA recognized EABP and IABP (Figure C). B cell activation (measured by Syk phosphorylation) assay indicated that AMPA-expressing B cells recognized EABP and (to a lesser extent) IABP, but not NABP (Figure D).
Conclusion: Acetylated bacterial proteins are potent antigens that can induce cross-reactive AMPA responses in mice, and can be recognized by human AMPA and AMPA-expressing B cells. This suggests that acetylated bacterial proteins could potentially be involved in the breach of tolerance in RA.
To cite this abstract in AMA style:
Volkov M, Kampstra A, van Schie K, Kwekkeboom J, Huizinga T, Toes R, van der Woude D. A Novel Mechanism Linking Mucosal Bacteria with Autoantibody Responses in RA: Acetylated Bacterial Lysate as a Model Antigen [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/a-novel-mechanism-linking-mucosal-bacteria-with-autoantibody-responses-in-ra-acetylated-bacterial-lysate-as-a-model-antigen/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-novel-mechanism-linking-mucosal-bacteria-with-autoantibody-responses-in-ra-acetylated-bacterial-lysate-as-a-model-antigen/