Session Information
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Rheumatoid Arthritis (RA) is a progressive and systemic autoimmune disorder associated with chronic and destructive inflammation of the joints. The hallmarks of RA are synovial cell proliferation, extensive neoangiogenesis and infiltration of numerous immune cells into the synovial tissue. In vitro approaches simulating RA synovial tissue are crucial in preclinical and translational research to expand our knowledge on RA human pathophysiology and to test new diagnostic and therapeutic applications. Here, we present the engineering of a spheroid-based model of RA synovial tissue which mimics the close interaction between cells and key pro-inflammatory mediators present in the inflamed synovium.
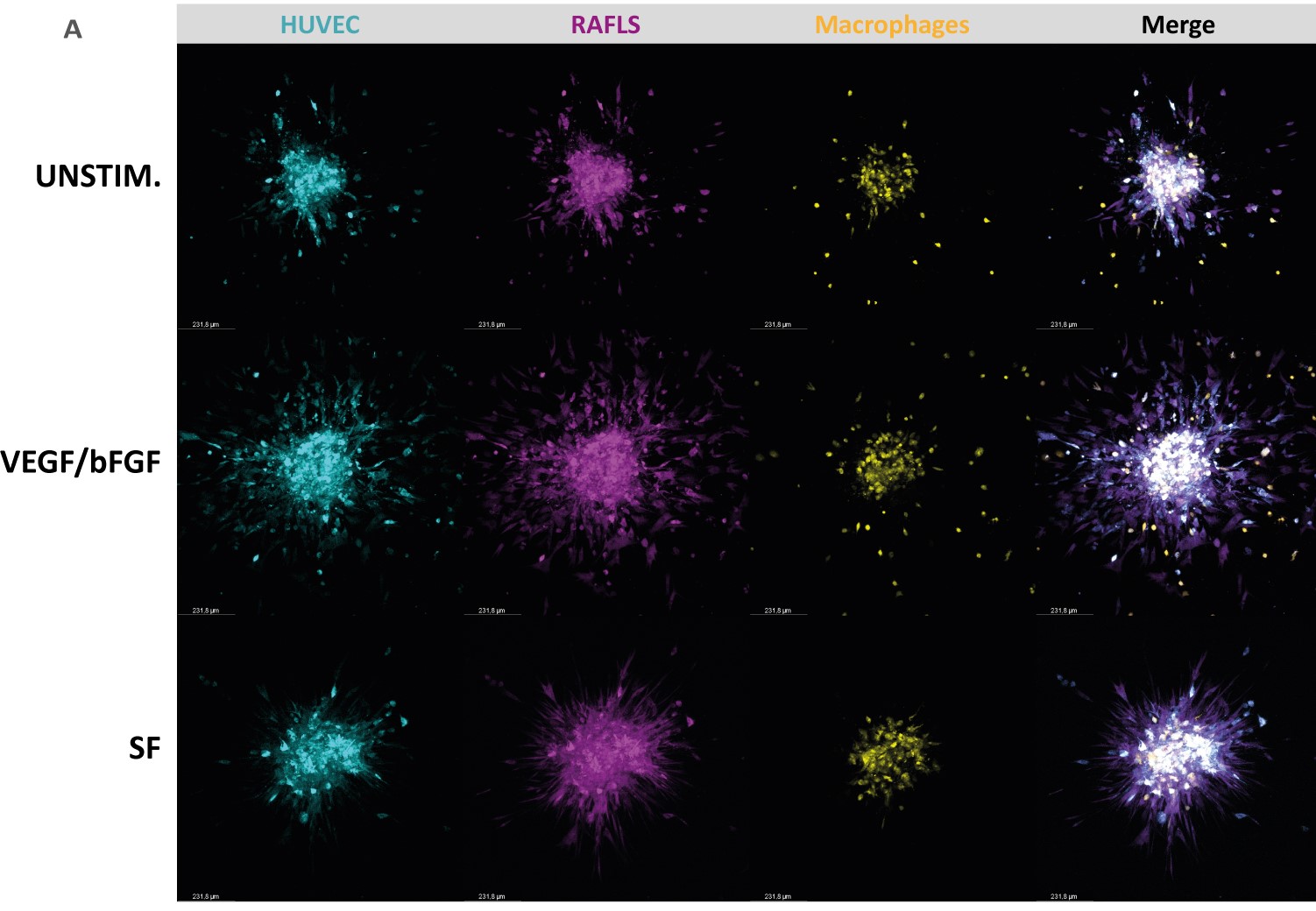
Methods: Monocyte-derived macrophages were cultured at different concentrations with RA fibroblast-like-synoviocytes (RAFLS) and endothelial cells (ECs) for 24 hours to allow for spheroid formation. Then, the spheroids were placed in a collagen-based matrix for 40 hours to study spheroid outgrowth in 3 dimensions. The spheroids were left unstimulated, or cultured in the presence of growth factors VEGF/bFGF or RA synovial fluid (SF). Spheroid outgrowth and cell migration were quantified for all conditions using confocal microscopy and a new quantification approach by machine learning (QuPath).
Results: Addition of macrophages to the previously established 3D model of RA angiogenesis consisting of ECs and RAFLS resulted in close interaction of macrophages with RAFLS and ECs within the spheroid structure. The number of macrophages that migrated out from the core increased with the initial macrophage input while slightly promoting spheroid outgrowth. The optimal ratio between RA-FLS, ECs and macrophages in our system was established accordingly as 1:2:0.8. Addition of growth factors (VEGF/bFGF) significantly promoted spheroid outgrowth compared to the unstimulated condition in the new model (p< 0.05). The presence of SF significantly enhanced cell containment of the ECs and macrophages within the core (p< 0.05).
Conclusion: We present a novel 3D-spheroid based model consisting of RA-FLS, ECs and macrophages that mimics the RA synovial tissue microenvironment. This model is useful to dissect the role of specific cell types in inflammatory responses in RA, to study specific signaling pathways involved in the disease pathogenesis and examine the potential of novel diagnostic (molecular imaging) and therapeutic compounds, including small molecule inhibitors and biologics.
To cite this abstract in AMA style:
Philippon E, van Rooijen L, van Hamburg J, Khodadust F, Van der Laken C, Tas S. A Novel 3D Model of Rheumatoid Arthritis Synovial Tissue Incorporating Fibroblasts, Endothelial Cells and Macrophages [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/a-novel-3d-model-of-rheumatoid-arthritis-synovial-tissue-incorporating-fibroblasts-endothelial-cells-and-macrophages/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-novel-3d-model-of-rheumatoid-arthritis-synovial-tissue-incorporating-fibroblasts-endothelial-cells-and-macrophages/