Session Information
Date: Sunday, November 7, 2021
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: SB4 is an etanercept biosimilar that obtained EU regulatory approval in 2016, and became available in Greece in 2018. Due to lack of published evidence about the use of etanercept biosimilars in the Greek setting, this study aims to generate real-world data on the impact of SB4 on patient-reported physical function and disease activity in RA patients.
Methods: BENEFICIAL is an ongoing 100-week non-interventional, multicenter, prospective study conducted in Greece, studying adult patients with moderate to severe active RA and inadequate response to conventional synthetic DMARDs. Patients were newly prescribed SB4 as monotherapy or combination therapy per the approved label in routine clinical practice. Patients who initiated SB4 >2 weeks before enrollment or who were previously treated with originator etanercept were excluded. Data are collected by routine clinical assessments, including tender/swollen joint count and physician’s global assessment of disease activity, acute phase reactants, and patient-reported outcomes (PROs) at baseline (SB4 treatment initiation), and at 24, 52 and 100 weeks post-baseline. PROs include the validated Greek Patient-Reported Outcomes Measurement Information System Physical Function Short Form 20a (PROMIS PF SF-20), and the patient’s global assessment of disease activity, joint pain intensity, and morning stiffness using visual analogue scales. We present the results of an interim analysis performed after all patients completed the 52-week observation period.
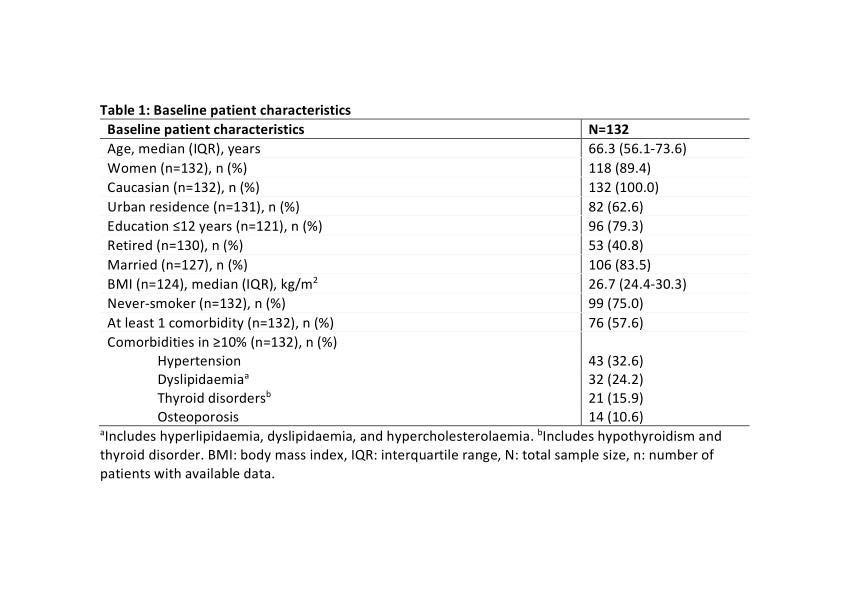
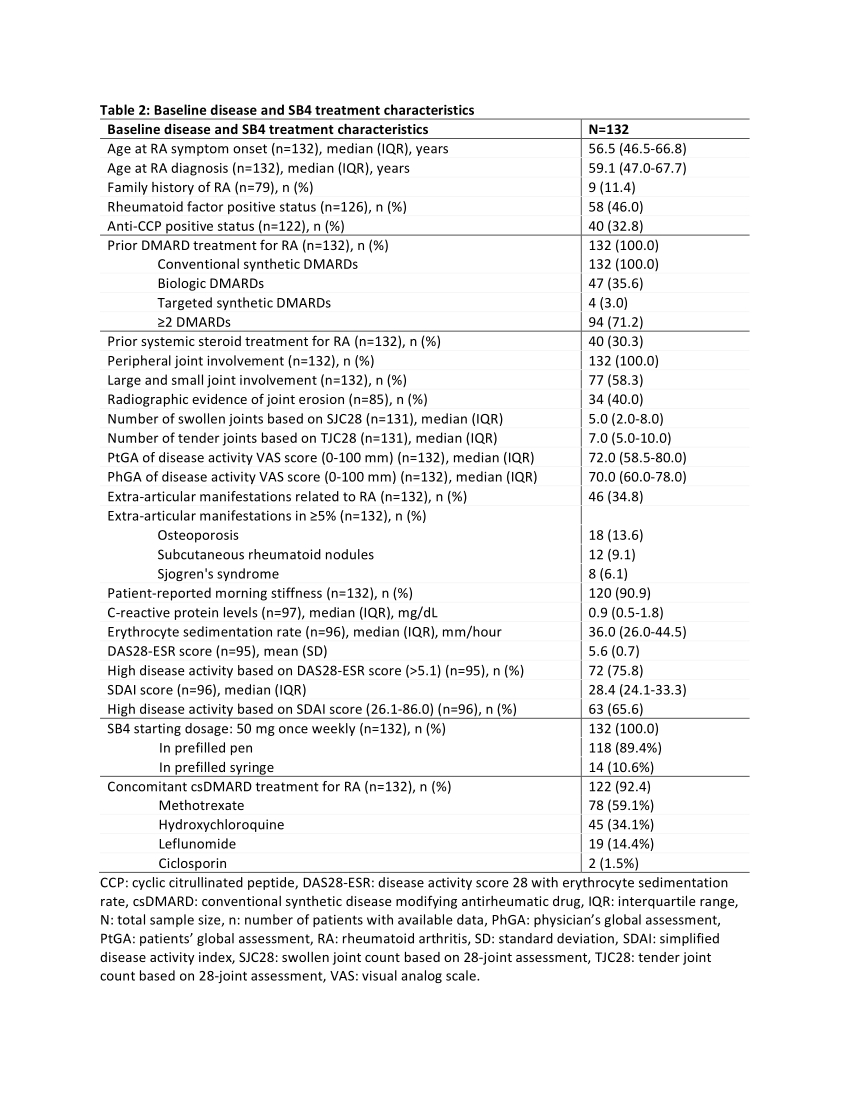
Results: Between 2/May/2019 and 9/Dec/2019, 132 eligible patients were enrolled in 9 rheumatology centers. Baseline characteristics are shown in Tables 1 and 2. Over a median (interquartile range; IQR) treatment duration of 50.1 (48.7-52.6) weeks, a median (IQR) of 50.0 (48.0-52.0) injections were received. The baseline median (IQR) PROMIS PF SF-20 total T-score was 28.7 (25.8-34.4).
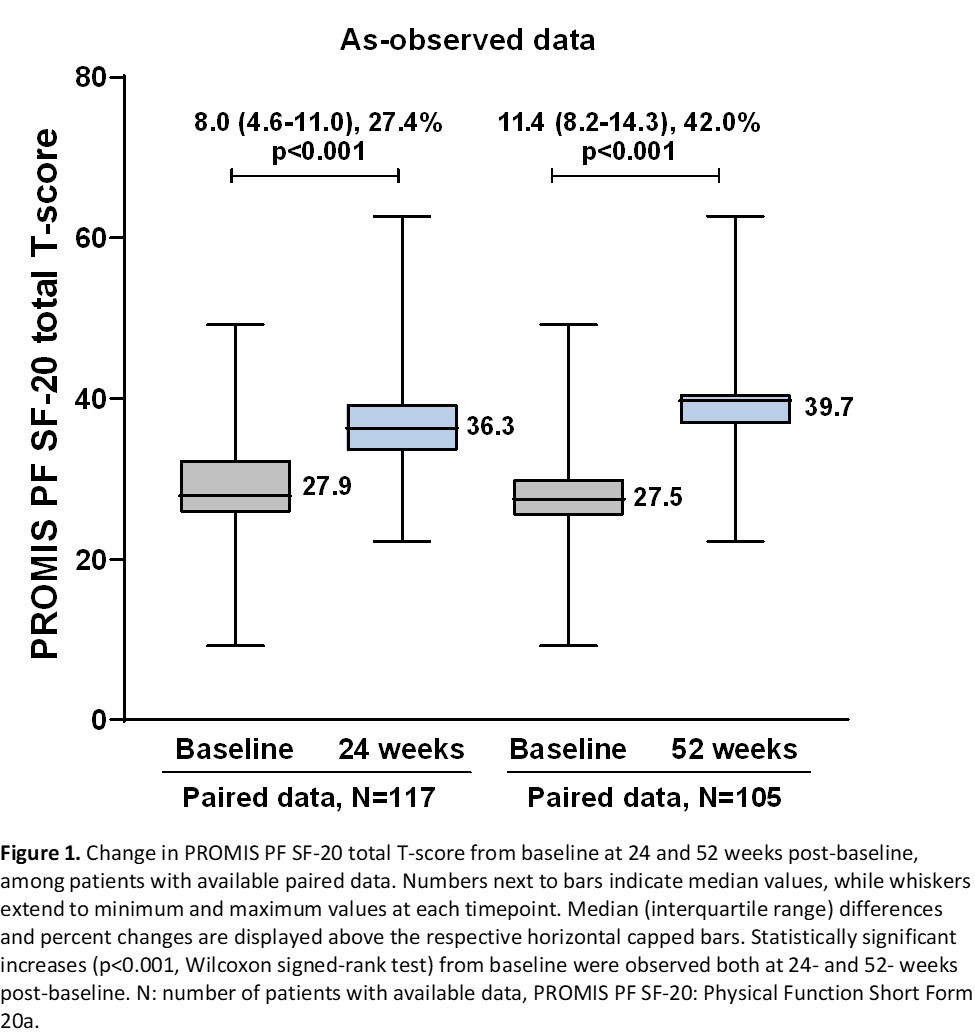
A clinically important improvement from baseline physical function (≥2 point-change in PROMIS PF SF-20 total T-score) was observed in 82.1% (95% CI: 75.1-89.0) and 92.4% (95% CI: 87.3-97.5) of the patients at 24 and 52 weeks, respectively. Median PROMIS PF SF-20 total T-score and all item scores significantly increased from baseline at both timepoints (Figure 1). The proportions of evaluable patients achieving low disease activity or remission based on Simplified Disease Activity Index (score ≤11) at 24 and 52 weeks were 42.9% (33/77) and 80.0% (56/70). Among evaluable patients, ACR20, ACR50, and ACR70 responses were attained by 68.1% (64/94), 22.3% (25/112) and 8.0% (9/113) of patients at 24 weeks, and by 86.8% (79/91), 58.1% (54/93) and 20.4% (19/93) at 52 weeks, respectively. The adverse event (AE) rate was 18.9% (25/132). AEs related to SB4 were reported for 3 (2.3%) patients (excluding events of ‘drug ineffective’) of whom one patient experienced 2 serious events.
Conclusion: These are the first real-world data on SB4 in a routine setting in Greece, indicating that the majority of patients in this study cohort demonstrated clinically meaningful improvement in their physical function and disease activity by 24 and 52 weeks post-initiation of SB4 for treatment of RA.
To cite this abstract in AMA style:
Athanassiou P, Bounas A, Boumpas D, Sidiropoulos P, Tsalapaki C, Garyfallos A, Grika E, Voulgari P, Kekki A, Anagnostopoulos Z, Karachalios G, Antonakopoulos N. A Non-Interventional Prospective Observational Study of Treatment with Etanercept Biosimilar (SB4) in Rheumatoid Arthritis in Real Life Clinical Practice; Interim Analysis of the ‘BENEFICIAL’ Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/a-non-interventional-prospective-observational-study-of-treatment-with-etanercept-biosimilar-sb4-in-rheumatoid-arthritis-in-real-life-clinical-practice-interim-analysis-of-the-beneficial/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-non-interventional-prospective-observational-study-of-treatment-with-etanercept-biosimilar-sb4-in-rheumatoid-arthritis-in-real-life-clinical-practice-interim-analysis-of-the-beneficial/