Session Information
Date: Tuesday, November 14, 2023
Title: (2352–2369) Systemic Sclerosis & Related Disorders – Clinical Poster III: Translational Science
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Systemic Sclerosis (SSc) is a rare autoimmune connective tissue disease characterized by inflammation and fibrosis. Treatment with mycophenolate mofetil (MMF), an inhibitor of lymphocyte guanine nucleotide synthesis that targets the enzyme inosine monophosphate dehydrogenase (IMPDH), is associated with clinical benefit in many SSc patients. Although MMF is thought to target lymphocytes exclusively, we have previously shown that MMF also reduces dermal myeloid cell numbers and CCL2 expression in the skin & sera of MMF-responsive SSc patients. Given macrophage plasticity, we hypothesized that, in addition to restricting macrophage skin infiltration, MMF might also mediate direct effects on dermal macrophage pro-fibrotic activation.
Methods: Clinical data from SSc patient cohorts were analyzed for myeloid gene signatures to identify changes in myeloid cell populations during MMF treatment. Primary human monocytes were cultured in clinically relevant serum concentrations of the active metabolite of MMF, mycophenolic acid (MPA), and analyzed for mRNA and surface marker expression, nitric oxide and cytokine production, cell viability and caspase activity in response to MPA. To assess MMF-mediated effects on pro-fibrotic macrophage activation, 3D tissue models of healthy and SSc skin were constructed and treated with or without MPA (0.5-10 µg/mL).
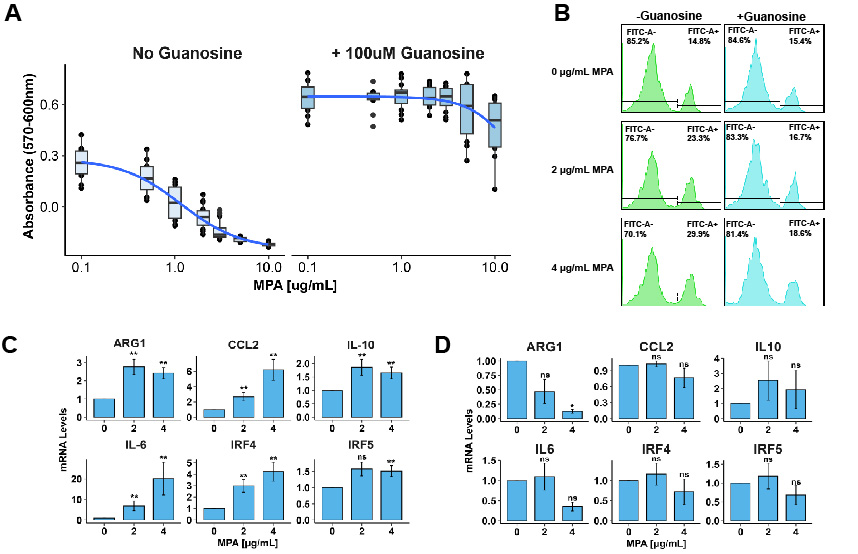
Results: SSc patients on MMF showed a significant decrease in myeloid cell populations in skin biopsies. The clinical target range for serum MPA levels are 1-3µg/mL for MMF patients; the IC50 of MPA in primary human monocytes in our experiments was found to be between 0.9-2.5 µg/mL (Fig. 1A), well within the clinical range. MPA dose-dependently induced apoptosis in primary human monocytes at concentrations as low as 0.5 µg/mL (Fig. 1B). Intriguingly, a subset of monocytes not killed by MPA upregulated surface expression and mRNA levels of markers associated with alternative activation (Fig. 1C). MPA treatment of monocytes in 2D co-cultures with fibroblasts and 3D fabricated skin models showed similar results. Addition of guanosine to cultures significantly attenuated the ability of MPA to induce apoptosis in and modulate activation of monocytes (Fig. 1D).
Conclusion: We now demonstrate here that MMF inhibits primary human macrophage viability and alters macrophage function. Our work suggests that IMPDH activity is required for macrophage viability and mediates alternative activation, as treatment with guanosine rescues cell death and significantly attenuates macrophage phenotype. We thus propose that clinical benefit observed with MMF in the treatment of fibrotic diseases such as SSc may be attributable, at least in part, to effects on myeloid cells.
To cite this abstract in AMA style:
Morris E, Parvizi R, Pioli P, Whitfield M. A Macrophage-Specific Mechanism for Mycophenolate Mofetil in the Treatment of Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/a-macrophage-specific-mechanism-for-mycophenolate-mofetil-in-the-treatment-of-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-macrophage-specific-mechanism-for-mycophenolate-mofetil-in-the-treatment-of-systemic-sclerosis/