Session Information
Date: Sunday, October 26, 2025
Title: Abstracts: Systemic Sclerosis & Related Disorders – Clinical I (0843–0848)
Session Type: Abstract Session
Session Time: 3:45PM-4:00PM
Background/Purpose: Mycophenolate Mofetil (MMF) is the most commonly prescribed immunosuppressive treatment for patients diagnosed with diffuse cutaneous systemic sclerosis (dcSSc). Here, we analyzed skin gene expression data for the largest longitudinal scleroderma cohort treated with MMF. By applying intrinsic molecular subtype classification to these skin gene expression data, we evaluated longitudinal changes in skin and other clinical outcomes during treatment, demonstrating an association between SSc molecular intrinsic subtypes and disease course during MMF treatment.
Methods: We analyzed longitudinal gene expression of 761 skin biopsies from a cohort of 201 scleroderma patients (based on ACR/EULAR 2013 criteria) with clinically active disease (in the treating physician’s opinion) and 57 healthy participants. Biopsies were obtained from lesional and non-lesional skin at baseline and at 6, 12, 24, and 36 months after start of MMF treatment initiation (Fig 1A). Clinical metadata including age, sex, ethnicity, autoantibodies, disease duration/stage, and modified Rodnan skin score (mRSS) were obtained. The intrinsic subtype of each sample was predicted using a machine learning classifier, distinct gene signatures were identified, and subtype enrichment was calculated.
Results: We identified improvers and non-improvers during MMF using 12-month mRSS post-treatment (Fig 1B). The inflammatory subtype had the highest mean mRSS, followed by the fibroproliferative and normal-like subtypes (Fig 1C). Within the MMF-treated cohort, most improvers (11 out of 21 improvers; 52%) were inflammatory. The majority of patients classified as non-improvers or stable were predicted to be normal-like (Fig 1D). The inflammatory subtype gene signature was enriched for immune cell activation pathways, whereas normal-like and fibroproliferative signatures were characterized by fatty acid metabolism, cell cycle, and other metabolic pathways (Fig 1E). The inflammatory enrichment scores correlate with markers of disease severity; negatively with disease duration and positively with mRSS, whereas the normal-like signature shows the opposite pattern (Fig. 2A). Early-stage patients ( < 1.5 years after disease onset) were shown to have a 46% likelihood of being classified as inflammatory, whereas patients with disease duration > 4.5 years had a 54% likelihood of being classified as normal-like. Patients with high mRSS were more likely to be classified as inflammatory (55%), while those with low mRSS were more likely classified as normal-like (Fig 2B). Lastly, the association between subtype and the clinical manifestations showed that both inflammatory and normal-like signatures were associated with disease phenotypes (Fig 2C). The fibroproliferative subtype remained stable over the course of disease progression in this cohort.
Conclusion: These analyses suggest a link between disease severity and the intrinsic molecular subtype signatures. Inflammatory patients were more active, had higher mRSS, and showed more improvement during MMF. These data suggest that the inflammatory and normal-like subtypes represent opposite phenotypic states and disease activity.
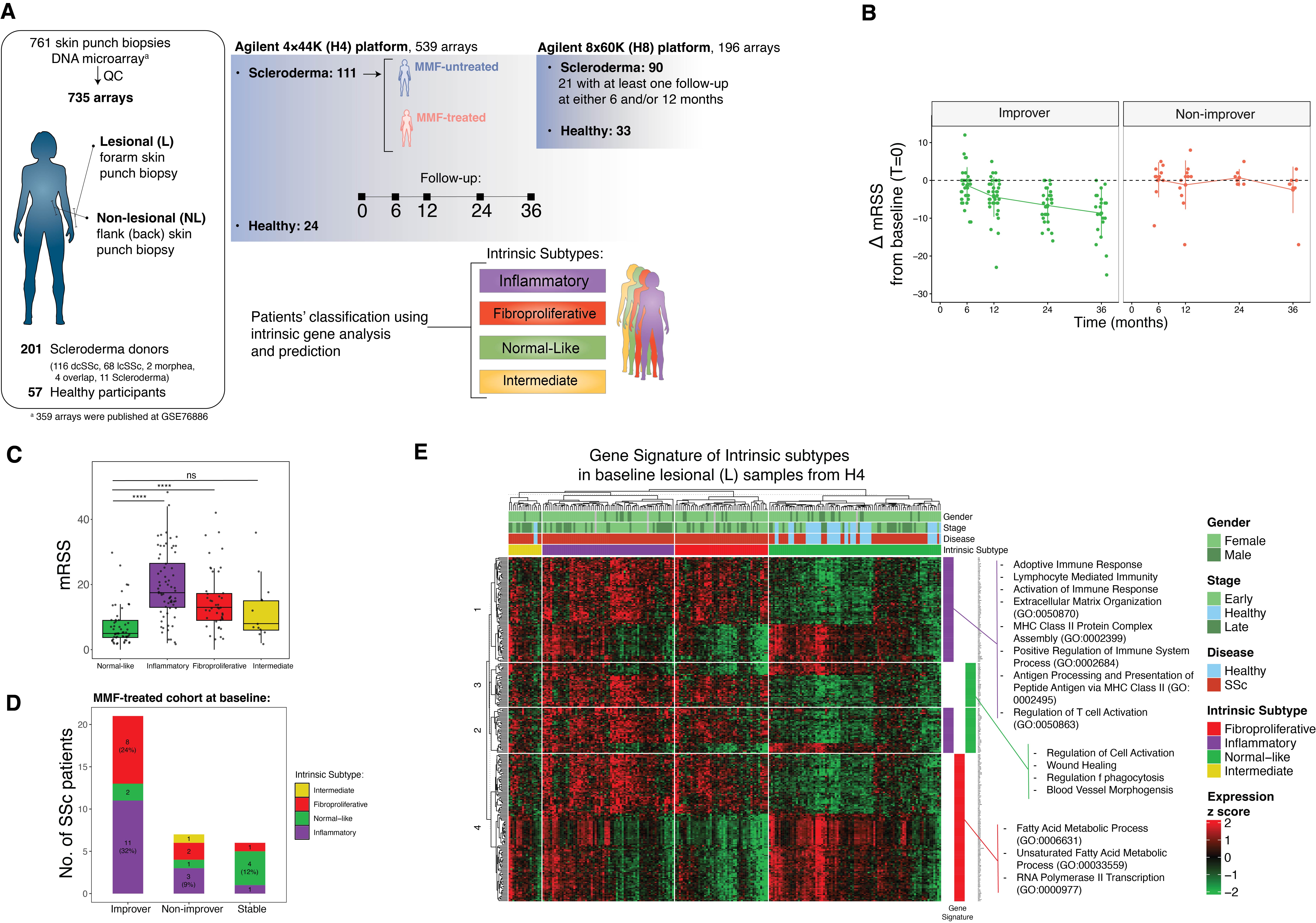 Figure 1. A) The schematic of the skin punch biopsy gene expression data set from lesional (forearm) and non-lesional (flank) of scleroderma and healthy donors that were processed in two DNA microarray platforms: Agilent 4x44K (H4) and Agilent 8x60K (H8). Intrinsic subtypes – inflammatory, fibroproliferative, normal-like and intermediate – were predicted for each skin sample. B) Change in mRSS from baseline for improvers in green and non-improvers in red. C) The mRSS levels for each intrinsic subtype for all patients at baseline. D) Distribution of subtypes for improvers, non-improvers, and stable patients who were treated with MMF. E) Heatmap showing intrinsic subtype gene signatures in baseline lesional skin samples from H4. The color bar on the right indicates gene expression levels associated with each intrinsic subtype signature. Representative Gene Ontology biological pathways enriched in each signature are shown next to the heatmap. dcSSc, diffuse cutaneous systemic sclerosis; lcSSc, limited cutaneous systemic sclerosis; MMF, Mycophenolate Mofetil; mRSS, modified Rodnan skin score.
Figure 1. A) The schematic of the skin punch biopsy gene expression data set from lesional (forearm) and non-lesional (flank) of scleroderma and healthy donors that were processed in two DNA microarray platforms: Agilent 4x44K (H4) and Agilent 8x60K (H8). Intrinsic subtypes – inflammatory, fibroproliferative, normal-like and intermediate – were predicted for each skin sample. B) Change in mRSS from baseline for improvers in green and non-improvers in red. C) The mRSS levels for each intrinsic subtype for all patients at baseline. D) Distribution of subtypes for improvers, non-improvers, and stable patients who were treated with MMF. E) Heatmap showing intrinsic subtype gene signatures in baseline lesional skin samples from H4. The color bar on the right indicates gene expression levels associated with each intrinsic subtype signature. Representative Gene Ontology biological pathways enriched in each signature are shown next to the heatmap. dcSSc, diffuse cutaneous systemic sclerosis; lcSSc, limited cutaneous systemic sclerosis; MMF, Mycophenolate Mofetil; mRSS, modified Rodnan skin score.
.jpg) Figure 2. A) The scatter plots and Pearson correlations of the inflammatory, fibroproliferative, and normal-like signatures over disease duration and mRSS for all SSc (red) and healthy (blue) samples. R-squared and p-values of Pearson correlations are depicted in the graph. (Disease duration was identified as the time of the biopsy to the onset of the disease, which was marked as first non-Raynaud’s symptom.) B) Distribution of SSc intrinsic subtypes across disease duration and mRSS. Both disease duration and mRSS values were categorized into four bins. The plot shows the number of patients in each subtype within these bins. C) Heatmap showing significant association between the intrinsic gene signatures and the clinical characteristics and manifestations.
Figure 2. A) The scatter plots and Pearson correlations of the inflammatory, fibroproliferative, and normal-like signatures over disease duration and mRSS for all SSc (red) and healthy (blue) samples. R-squared and p-values of Pearson correlations are depicted in the graph. (Disease duration was identified as the time of the biopsy to the onset of the disease, which was marked as first non-Raynaud’s symptom.) B) Distribution of SSc intrinsic subtypes across disease duration and mRSS. Both disease duration and mRSS values were categorized into four bins. The plot shows the number of patients in each subtype within these bins. C) Heatmap showing significant association between the intrinsic gene signatures and the clinical characteristics and manifestations.
To cite this abstract in AMA style:
Parvizi R, Gong Z, Field N, Jarnagin H, Popovich D, Yang M, Aren K, Carns M, Goldberg I, Chung L, Goh V, McMahan Z, Wood T, Khanna D, Hinchcliff M, Whitfield M. A Longitudinal Transcriptomic Study of Mycophenolate Mofetil in Systemic Sclerosis Skin with Clinical and Molecular Stratification [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/a-longitudinal-transcriptomic-study-of-mycophenolate-mofetil-in-systemic-sclerosis-skin-with-clinical-and-molecular-stratification/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-longitudinal-transcriptomic-study-of-mycophenolate-mofetil-in-systemic-sclerosis-skin-with-clinical-and-molecular-stratification/
