Session Information
Date: Sunday, November 17, 2024
Title: Systemic Sclerosis & Related Disorders – Basic Science Poster I
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Mitochondria are essential eucaryotic cell organelles with several bacterial features, such as a double-stranded circular genome with hypomethylated CpG areas (1). A fundamental role of mitochondria in autoimmunity was recently demonstrated. In brief, mitochondrial ROS participate in the formation of neutrophil extracellular traps (NETs) (2), while extrusion of cell-free mitochondria and highly oxidised interferogenic mitochondrial DNA (ox-mtDNA) causes autoimmune disease in an animal model (3, 4). In other connective tissue diseases, plasma mtDNA is a diagnostic biomarker, also reliable in the monitoring of disease activity (5), while platelets might also contribute as a major source of circulatory mtDNA.
The present study aimed to explore the cellular mechanisms controlling the inflammatory responses triggered by mtDNA in systemic sclerosis (SSc) (6).
Methods: Total DNA was isolated from plasma samples of healthy individuals and SSc patients. Copy numbers were analysed for mitochondrial (mt) DNA (ATP-6) and nuclear (n) DNA (GAPDH) abundance by qPCR. mtDNA was isolated from HC and SSc patients. Patients were stratified based on mtDNA levels. Neutrophils and platelets were isolated from EDTA and citrate blood, respectively; cells were incubated with SSc patients’ plasma and mtDNA, and NET formation was assessed by SytoxGreen staining. Platelets were tested for mtDNA release propensity. DNA oxidation was evaluated by MitoSOX Red staining in vitro and 8-OHdG ELISA of patient plasma. Plasma IFN type 1 was measured by ELISA. Platelet activation was assessed by IF staining in vitro and ELISA for CXCL4.
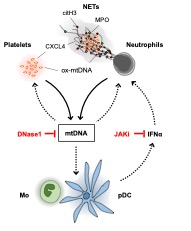
Results: SSc patients’ plasma contains elevated levels of highly oxidized mtDNA. Healthy donors’ blood neutrophils incubated with plasma from patients with SSc exert enhanced NET formation, and NETs are decorated with oxidised mtDNA. SSc platelets contain and release oxidized mtDNA, eliciting increased NET formation and mtDNA release from activated platelets. Moreover, mtDNA amounts correlate with CXCL4 and type I IFN levels in SSc patients’ plasma. Finally,formation of NETs and ox-mtDNA extrusion are conveyed through the type I IFN pathway.
Conclusion: SSc plasma is characterized by an abundance of mtDNA that promotes positive feedback loops driving its release by NETs and platelets. Moreover, mtDNA is particularly interferogenic and may contribute to tissue damage and fibrosis in SSc (Figure 1).
To cite this abstract in AMA style:
Giaglis S, Kyburz D, Walker U. A Feedback Loop Involving mtDNA, Platelets and Neutrophil Extracellular Traps Amplifies Inflammation in Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-feedback-loop-involving-mtdna-platelets-and-neutrophil-extracellular-traps-amplifies-inflammation-in-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-feedback-loop-involving-mtdna-platelets-and-neutrophil-extracellular-traps-amplifies-inflammation-in-systemic-sclerosis/