Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: The presentation of MHC-peptide complexes to T lymphocytes via antigen-presenting cells (APC) is a crucial step in the initiation of immune responses. Dendritic cells (DC) are potent APC with remarkable plasticity that are classically divided into DC1 (141+), DC2 (1c+) and plasmacytoid (pDC) subsets. Decreases in these classic blood DC occur in patients suffering from autoimmune conditions including RA. We have previously reported that in RA, peripheral decreases of DC2 are strongly associated with clinical RA features including measures of cardiovascular disease (CVD), the main driver of RA related mortality. We reasoned that — given the importance of DC in immune activation — their apparent decrease might be more adequately explained by phenotypic alteration rather than ‘true’ disappearance. We hypothesized that this highly plastic compartment under inflammatory conditions might harbor alternative phenotypes that lack classic combinations of DC markers while retaining potential APC function (HLA-DR).
Methods: Patients with RA and SLE — two prototypical autoimmune rheumatic diseases (ARD) — and healthy donors (HD) underwent immunophenotyping; to capture the presumed candidate APC we first gated all viable HLA-DR+ cells. By eliminating the lymphoid lineage (CD3–,CD19–) we created an HLA-DR+Lin– superset containing classic DCs, monocytes and candidate APC. We next classified HLA-DR+Lin– cells into established non-lymphoid APC [classic DC and Monocyte] by using a combination of t-distributed stochastic neighbor embedding (t-SNE) and manual annotation. HLA-DR+Lin– cells that did not meet established APC criteria we designate candidate APC. To screen for common characteristics across patients within this subset we performed Uniform Manifold Approximation and Projection (UMAP).
Results: Strikingly, candidate APC were higher in ARD (n=21) (7.3% vs. 3.9%; [median %DR+, Kruskall-Wallis tests), particularly SLE (11.8% vs 3.9%; p=0.01). UMAP of RA cAPC identified a dominant cluster of CD45RA+CD14lo as a feature present across RA samples. CD14intCCR2+ cAPC represented a second major cluster. Confirming traditional gating approaches, DC classical subsets were decreased in ARD when compared with HD (n=10): DC1 0.1% vs 0.4% (p=0.01), DC2 2.6% vs 5.3% (p=0.03) and pDC 1.5% vs 2.9% (p=0.03). Classical (CD14+CD16–) monocytes were not different (p=0.19) whereas intermediate monocytes (CD14+CD16+) were higher in RA than SLE and HD (13.2% vs. 8.9% vs. 6.2; p=0.01).
Conclusion: Using a combination of dimensionality reduction techniques on an unbiased HLA-DR+Lin– APC superset we observed the appearance of HLA-DR+ candidate APC. These cells do not conform to traditional APC gating definitions and were increased in ARDs relative to healthy controls. Their dominant cluster showed widespread expression of CD45RA suggesting, despite lack of CD1c expression, a developmental relationship with DC2. CD14/CCR2 co-expression was seen in three patients, supporting a relationship to monocyte-derived DC in these individuals. The novel subsets may be implicated in the pathophysiology of ARDs including RA associated cardiovascular disease, potentially via anomalous presentation of self-antigen to T lymphocytes.
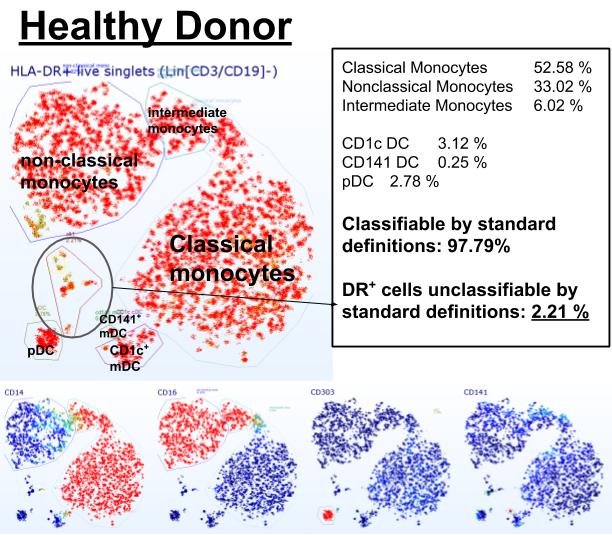 Figure 1. Example of t-distributed Stochastic Neighbor Embedding (t-SNE) guided manual annotation of high-dimensional flow cytometry data of a healthy blood donor. Non-lymphoid cells with antigen-presenting potential (DR positive, Lin negative) can be classified into established lineages in 97.79%. Bottom row shows color coded overlays of expression intensity (red positive, blue negative) of select monocyte and DC markers (CD14, CD16, CD303, CD141). Dimensions not shown: CCR2, XCR1, CD1c, CD11c, CD45RA, CD56, CD163, CD123, CD172a (SRPα).
Figure 1. Example of t-distributed Stochastic Neighbor Embedding (t-SNE) guided manual annotation of high-dimensional flow cytometry data of a healthy blood donor. Non-lymphoid cells with antigen-presenting potential (DR positive, Lin negative) can be classified into established lineages in 97.79%. Bottom row shows color coded overlays of expression intensity (red positive, blue negative) of select monocyte and DC markers (CD14, CD16, CD303, CD141). Dimensions not shown: CCR2, XCR1, CD1c, CD11c, CD45RA, CD56, CD163, CD123, CD172a (SRPα).
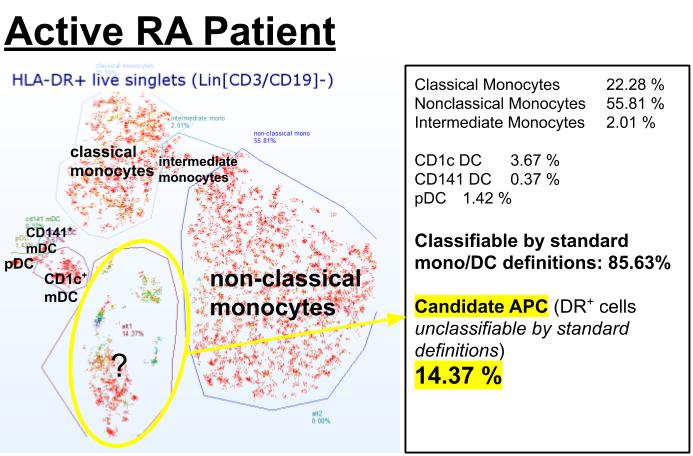 Figure 2. Example of t-SNE guided manual annotation of flow cytometry data of an active RA patient (CDAI 29), showing the emergence of 14.37% of phenotypes with antigen-presenting potential (HLA-DR positive) which are unclassifiable using standard definitions (candidate APC).
Figure 2. Example of t-SNE guided manual annotation of flow cytometry data of an active RA patient (CDAI 29), showing the emergence of 14.37% of phenotypes with antigen-presenting potential (HLA-DR positive) which are unclassifiable using standard definitions (candidate APC).
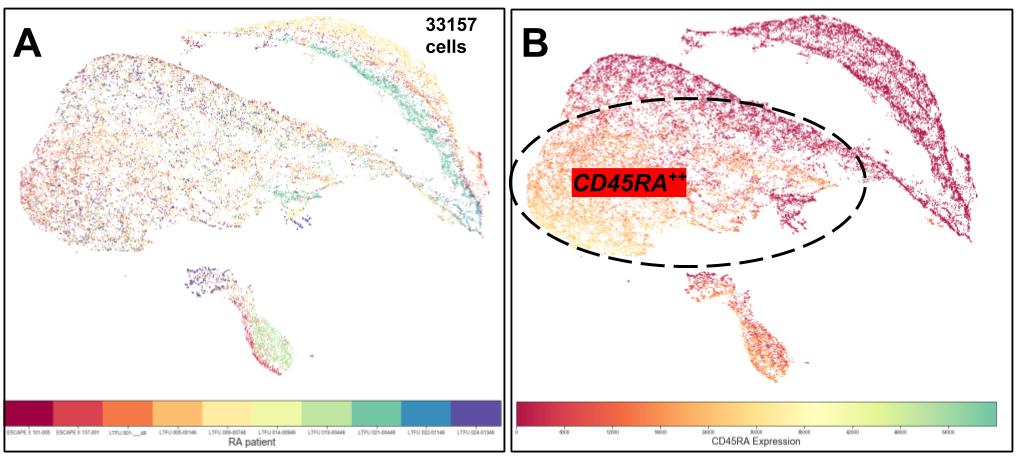 Figure 3. Uniform Manifold Approximation and Projection (UMAP) using 33157 candidate APC which were concatenated from 10 RA patients. A. Color coded by donor, showing three main clusters with cells derived from multiple donors. B. Same UMAP Heat-coded overlay showing expression of CD45RA in majority of the dominant cluster. Dimensions not shown: CD14, CD16, CD141, 303, CCR2, XCR1, CD1c, CD11c, CD45RA, CD56, CD163, CD123, CD172a (SRPα).
Figure 3. Uniform Manifold Approximation and Projection (UMAP) using 33157 candidate APC which were concatenated from 10 RA patients. A. Color coded by donor, showing three main clusters with cells derived from multiple donors. B. Same UMAP Heat-coded overlay showing expression of CD45RA in majority of the dominant cluster. Dimensions not shown: CD14, CD16, CD141, 303, CCR2, XCR1, CD1c, CD11c, CD45RA, CD56, CD163, CD123, CD172a (SRPα).
To cite this abstract in AMA style:
Geier C, Giles J, Gaines S, Depender C, Bathon J, Winchester R. A Combination of Dimensionality Reduction Techniques Reveals Novel HLA-DR+ ‘Candidate’ Antigen-Presenting Cell Subsets (cAPC) in Patients with Rheumatoid Arthritis (RA) [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/a-combination-of-dimensionality-reduction-techniques-reveals-novel-hla-dr-candidate-antigen-presenting-cell-subsets-capc-in-patients-with-rheumatoid-arthritis-ra/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-combination-of-dimensionality-reduction-techniques-reveals-novel-hla-dr-candidate-antigen-presenting-cell-subsets-capc-in-patients-with-rheumatoid-arthritis-ra/
