Session Information
Session Type: Late-Breaking Abstract Session
Session Time: 8:00AM-8:15AM
Background/Purpose: Over 50% of RA patients require multiple b/tsDMARD classes due to inadequate or lost response. Rosnilimab, an investigational monoclonal antibody that selectively targets and depletes pathogenic T cells of interest (i.e., PD-1high Tph/Tfh, Teff), is upstream of several clinically validated targets in RA and offers potential advantages as a new treatment modality.
Methods: This multicenter, randomized, double-blind, placebo-controlled Phase 2B trial (NCT06041269) enrolled adults with moderate to severe (≥6 tender and ≥6 swollen joints) seropositive RA on concurrent csDMARDs and < 3 previous b/tsDMARDs. Participants were randomized 1:1:1:1 to receive SC rosnilimab 100mg Q4W, 400mg Q4W, 600mg Q2W or placebo. Primary endpoint was at Wk 12. Participants randomized to rosnilimab were required to achieve CDAI LDA (≤10) at Wk 14 to continue in the Blinded All-Active Treatment Period through Wk 28, with subsequent off-drug follow-up to Wk 38.
Results: 424 participants were randomized; 41% had prior b/tsDMARD exposure; 95.0% and 86.6% were RF and CCP positive, respectively. At baseline, participants had a mean DAS28-CRP of 5.6, mean CDAI of 37.7 and mean CRP of 18mg/L.
The primary endpoint, mean change from baseline in DAS28-CRP at Wk 12, was met by all rosnilimab doses (-2.06, -2.12, -2.06 in 100mg, 400mg and 600mg arms) versus placebo (-1.69) (p < 0.01). At Wk 12, all rosnilimab dose arms had significantly greater changes in ACR20 and mean change in CRP versus placebo. ACR 50, ACR70, CDAI LDA and patient reported outcomes (PROs) consistently demonstrated higher responses over placebo (Fig 1a).
220 (69%) rosnilimab-treated participants reached CDAI LDA at Wk 14 and were eligible to continue into the All-Active Period. Those not eligible for the All-Active Period were imputed as nonresponders at all future visits, although many would be considered responders by other criteria (60% achieved ACR20 and 18% achieved ACR50). In the All-Active cohort, response rates substantially increased from Wk 12 to Wk 28 for secondary endpoints (ACR 50,70, LDA) and PROs (HAQ, pain VAS) (Fig 1a). After Wk 26, during the 12-14 week off drug period, responses were generally maintained (Fig 1b). Results were similar in b/tsDMARD naïve and experienced participants.
Translational data showed rapid depletion ( >90%) of pathogenic T cells of interest in blood with preservation of the total T cell numbers through the 28 Wk treatment period, consistent with the durability of clinical outcomes (Fig 2a). Synovial biopsies at Wk 6 (n = 24 evaluable pairs) demonstrated similar >90% depletion of pathogenic T cells of interest (Fig 2b) and broad downregulation of gene signatures including T cell, B cell, and myeloid activation.
Rosnilimab was well tolerated. 28/424 (7%) participants discontinued prior to Wk 12. 8/220 (4%) participants discontinued during the All-Active Period. No deaths or malignancies were reported. No serious or severe infections leading to treatment withdrawal were reported in rosnilimab-treated participants (Table 1).
Conclusion: Rosnilimab selectively and potently depletes pathogenic T cells in RA leading to clinically meaningful and durable responses. These data support further exploration of rosnilimab in RA.
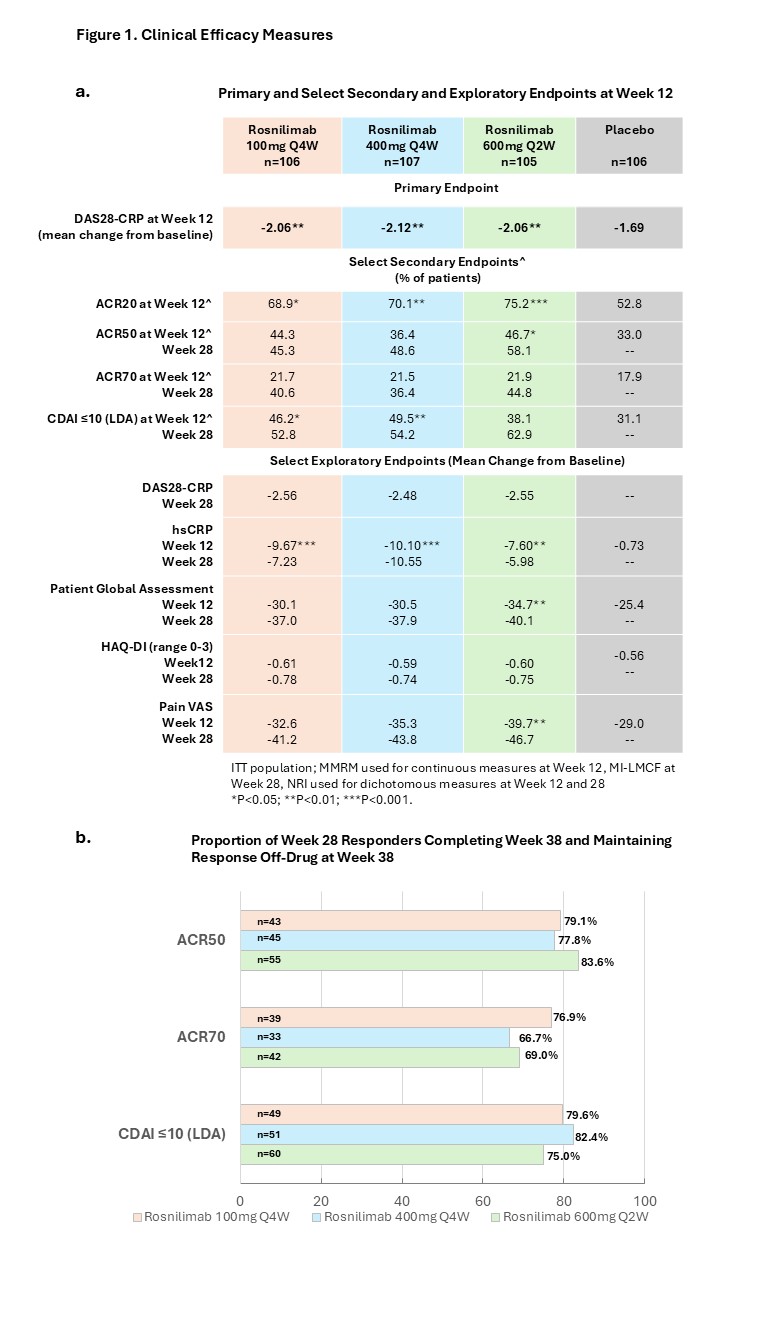 Figure 1. Clinical Efficacy Measures. a) Primary, Selected Secondary and Exploratory Endpoints at Week 12 and Week 28; MMRM = Mixed model repeated measures; used for continuous measures at week 12. MI-LMCF = Multiple imputations with last mean carried forward; used for continuous efficacy measures at Week 28. NRI = nonresponder imputation (NRI); used for dichotomous endpoints at Week 12 and 28. *p < 0.05; **p < 0.01; ***p < 0.001. b) Proportion of Week 28 Responders Completing Week 38 and Maintaining Response Off-Drug at Week 38.
Figure 1. Clinical Efficacy Measures. a) Primary, Selected Secondary and Exploratory Endpoints at Week 12 and Week 28; MMRM = Mixed model repeated measures; used for continuous measures at week 12. MI-LMCF = Multiple imputations with last mean carried forward; used for continuous efficacy measures at Week 28. NRI = nonresponder imputation (NRI); used for dichotomous endpoints at Week 12 and 28. *p < 0.05; **p < 0.01; ***p < 0.001. b) Proportion of Week 28 Responders Completing Week 38 and Maintaining Response Off-Drug at Week 38.
.jpg) Figure 2. Translational Data. a) Peripheral Flow T Cell Subsets Over Time: Percent Change from Baseline; b) Synovial Biopsy Immunohistochemistry Images
Figure 2. Translational Data. a) Peripheral Flow T Cell Subsets Over Time: Percent Change from Baseline; b) Synovial Biopsy Immunohistochemistry Images
.jpg) Table 1. Safety: Baseline Through Week 38 (End of Follow Up)
Table 1. Safety: Baseline Through Week 38 (End of Follow Up)
To cite this abstract in AMA style:
Graf J, Archer A, Kovalenko S, Kolossa K, Serpa J, Kobakhidze T, Cepoi D, Everding A, Pitzalis C, Aversa C, Dahl M, Hafez M, Lizzul P, Raina P, Randazzo B, Ren Y, Saikali K, Sibley C, Burmester G, GOTTENBERG J, McInnes I, Mysler E, Simon L, Smolen J, Sparks J, van Vollenhoven R, Weinblatt M, Emery P. Rosnilimab, a Selective and Potent Depleter of Pathogenic T Cells, Demonstrates Efficacy, Safety, and Translational Proof of Mechanism in a Rheumatoid Arthritis Phase 2B Trial [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/rosnilimab-a-selective-and-potent-depleter-of-pathogenic-t-cells-demonstrates-efficacy-safety-and-translational-proof-of-mechanism-in-a-rheumatoid-arthritis-phase-2b-trial/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rosnilimab-a-selective-and-potent-depleter-of-pathogenic-t-cells-demonstrates-efficacy-safety-and-translational-proof-of-mechanism-in-a-rheumatoid-arthritis-phase-2b-trial/
