Session Information
Date: Wednesday, October 29, 2025
Title: Abstracts: Rheumatoid Arthritis – Treatment II: Phenotyping and Personalization (2639–2644)
Session Type: Abstract Session
Session Time: 10:00AM-10:15AM
Background/Purpose: Emerging data show the potential of T-cell engagers (TCEs) for deep B cell depletion to treat autoimmune diseases. Cizutamig is a purposefully designed BCMAxCD3 TCE based on the FIT-Ig® platform and an optimized CD3 binding affinity to reduce cytokine release while maintaining killing of BCMA-expressing B cells, plasmablasts and plasma cells. This study aimed to evaluate the translatability of TCE design from preclinical data to clinical activity in patients.
Methods: Previous in vitro methods on plasma cell killing, T cell activation, and cytokine release comparing cizutamig to benchmark BCMA TCEs have been previously described. To assess in vivo preclinical activity, immunodeficient mice reconstituted with peripheral blood mononuclear cells (PBMCs) from a patient with systemic lupus erythematosus (SLE) were treated with 3 weekly doses of vehicle, isotype (1 mg/kg), or 0.1, 0.3, or 1 mg/kg of cizutamig (n=10 per group). Levels of human serum IgG, anti-dsDNA antibodies, splenic B cells and kidney immune complexes were measured. A Phase 1 dose escalation study to assess safety and efficacy was completed in patients with relapsed/refractory multiple myeloma (RRMM) in which cizutamig was administered weekly with step-up dosing. A patient with rheumatoid arthritis (RA) refractory to 5 targeted synthetic and biologic DMARDs including rituximab was the first non-oncology patient treated with weekly doses of cizutamig.
Results: In vitro, cizutamig showed comparable T cell dependent cytotoxicity of BCMA-expressing cell lines with lower cytokine release compared to benchmark BCMA TCEs (Fig 1). In the SLE mouse model, cizutamig showed complete depletion of serum IgG and anti-dsDNA antibodies, splenic B cells, and IgG deposits in the kidneys (Fig 2). In the Phase 1 study, 40 RRMM patients were treated with cizutamig at doses from 0.2 mg to 300 mg. For those who received ≥ 60mg dose, the objective response rate was 66% (12 of 18 patients) with minimal residual disease achieved in 42% of responders. Across all doses, only Grade 1 (18%) or Grade 2 (8%) cytokine release syndrome (CRS) per ASTCT criteria was seen with a median onset time and duration of 1 day (range 1-2) and 3 days (range 1-6), respectively. No immune effector cell-associated neurotoxicity syndrome (ICANS) was observed. In the refractory RA patient, after 3 weeks of treatment, near complete depletion of serum plasma light chains, bone marrow CD138+ plasma cells, and lymph node CD19+ and CD20+ B cells and CD138+ plasma cells were observed (Fig 3). Clinical response was achieved with DAS28 score decreasing from 6.3 to 2.7 units 5 weeks after treatment initiation. No CRS or ICANS was observed.
Conclusion: Cizutamig was designed to mitigate cytokine release while preserving cytotoxicity of BCMA-expressing cells. Preclinical and clinical data demonstrate a differentiated safety profile with signs of clinical activity in RRMM patients and a patient with refractory RA. Further clinical evaluation of cizutamig in autoimmune diseases is being explored.
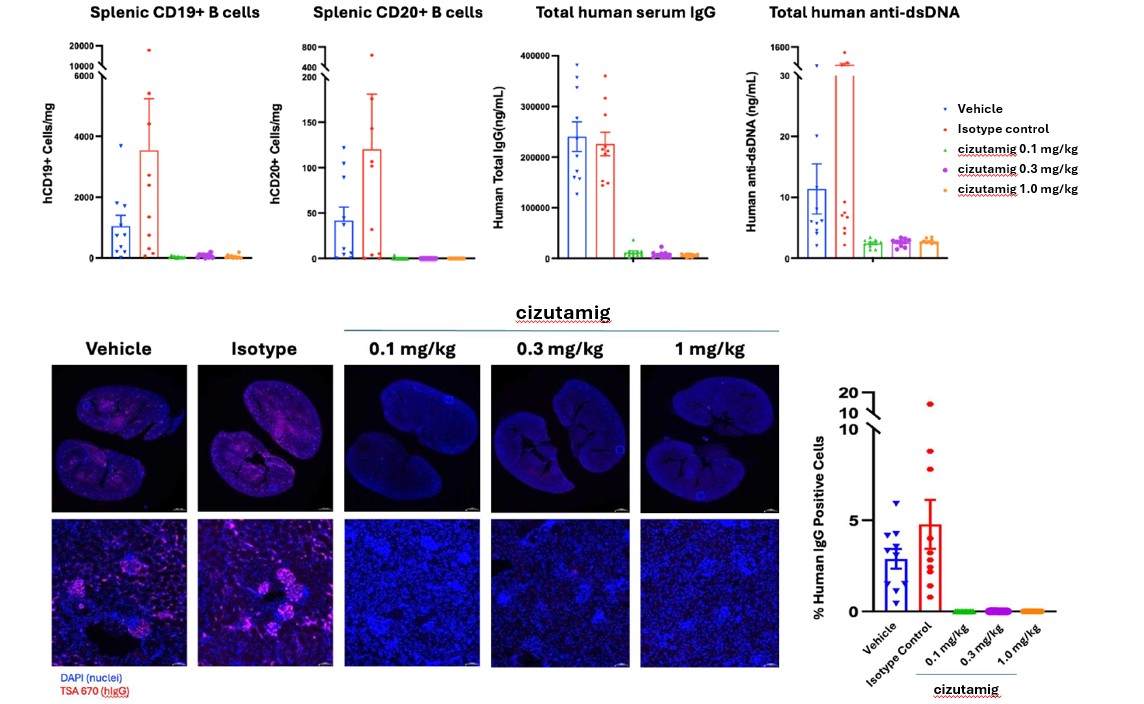 Fig 1. T cell mediated killing and cytokine release by healthy donor PBMCs co-cultured with BCMA-expressing cell lines in the presence of cizutamig or benchmark BCMAxCD3 TCEs (elranatamab, teclistamab).
Fig 1. T cell mediated killing and cytokine release by healthy donor PBMCs co-cultured with BCMA-expressing cell lines in the presence of cizutamig or benchmark BCMAxCD3 TCEs (elranatamab, teclistamab).
.jpg) Fig 2. Cizutamig mediated elimination of human splenic B cells, serum IgG, anti-dsDNA, and glomerular immune deposits in SLE patient PBMC humanized mouse model.
Fig 2. Cizutamig mediated elimination of human splenic B cells, serum IgG, anti-dsDNA, and glomerular immune deposits in SLE patient PBMC humanized mouse model.
.jpg) Fig 3. Cizutamig mediated depletion of B cells and plasma cells in the bone marrow and lymph node 3 weeks from start of treatment (BL: baseline, FSC-A: forward scatter area, SSC-A: side scatter area).
Fig 3. Cizutamig mediated depletion of B cells and plasma cells in the bone marrow and lymph node 3 weeks from start of treatment (BL: baseline, FSC-A: forward scatter area, SSC-A: side scatter area).
To cite this abstract in AMA style:
Grieshaber-Bouyer R, Haddon J, Raimondo M, Tur C, Böltz S, Dashi T, Hagen M, Wirsching A, Rathe T, Wu X, Wung P, Kim F, Zhu Y, Song K, Lu T, Schett G. Cizutamig, a BCMA T-cell engager: preclinical to clinical translation of design optimization for the treatment of autoimmune diseases [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/cizutamig-a-bcma-t-cell-engager-preclinical-to-clinical-translation-of-design-optimization-for-the-treatment-of-autoimmune-diseases/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cizutamig-a-bcma-t-cell-engager-preclinical-to-clinical-translation-of-design-optimization-for-the-treatment-of-autoimmune-diseases/
