Session Information
Date: Tuesday, October 28, 2025
Title: (2470–2503) Systemic Sclerosis & Related Disorders – Clinical Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Gastrointestinal (GI) involvement is among the most frequent organ manifestations in systemic sclerosis (SSc), yet the timing of presentation of GI manifestations remains incompletely understood. In this retrospective analysis, we aimed to describe the baseline prevalence and the incidence of new GI disease manifestations over time in a real-life, large SSc cohort.
Methods: We included patients from our center’s cohort fulfilling the ACR/EULAR 2013 criteria for SSc or the VEDOSS criteria. Electronic medical records were searched for data regarding the presence and the time of detection of specific GI diagnoses. We focused on esophageal disease (defined as at least one of: reflux, dysphagia, or aperistalsis), gastroparesis, gastric antral vascular ectasia (GAVE), small intestinal bacterial overgrowth (SIBO), chronic intestinal pseudo-obstruction (CIPO), chronic constipation, and chronic diarrhea. In addition, data of the University of California at Los Angeles/Scleroderma Clinical Trials Consortium Gastro-Intestinal Tract instrument 2.0 (GIT 2.0) questionnaire were also collected at each annual visit, when available. Based on the GIT 2.0 score, GI disease burden was categorized at baseline into 3 groups: (1) none to mild, (2) moderate, and (3) severe-to-very severe symptoms (Khanna D. et al, Arthritis Rheum 2009). We assessed baseline prevalence of GI diagnoses and used survival curves to investigate the timing of GI diagnoses from disease onset, defined as time from the first non-Raynaud’s sign or symptom.
Results: We included 658 patients: 83.6% females, mean age 55.4 (SD 15.0) years, 566 (86%) with available GIT 2.0; additional baseline clinical data are displayed in Table 1. At the first visit, 318 (48.3%) patients already presented with GI manifestations or diagnoses. Of these, the most common was esophageal disease (45.0%), while all other clinically relevant GI manifestations had an initial prevalence of < 4%. According to the GIT 2.0 categories, most patients had none to mild symptoms, while 102 (15.5%) had moderate and 30 (4.6%) had severe-to-very severe GI disease.Over a mean follow-up time of 3.3 (SD 3.2) years, additional patients developed new GI complications, with the cumulative prevalence increasing to 65% at last follow-up. In particular, esophageal disease increased to 61.1%, Barrett’s esophagus from 3.8% to 7.9%, GAVE from 1.4% to 4.3%, SIBO from 0.8% to 7.0%, anal incontinence from 2.3% to 7.6% and GI bleeding from 1.8% to 5.3%. Only 4 patients, already diagnosed with SIBO, developed CIPO during follow-up.Focusing on patients with known disease duration from SSc onset [n=476 (72%), median 3.6 (IQR 1.2-9.1 years)], we observed that esophageal involvement tended to appear earlier (median time from disease onset: 7.5 years), while others like Barrett’s esophagus or SIBO occurred later (Figure 1 A to E).
Conclusion: GI involvement in SSc is frequent and dynamic, with distinct time patterns for individual complications. Early manifestations such as esophageal disease precede more severe complications like GAVE and SIBO. Longitudinal tracking of GI disease in SSc provides important insights into its natural history and may inform anticipatory management strategies.
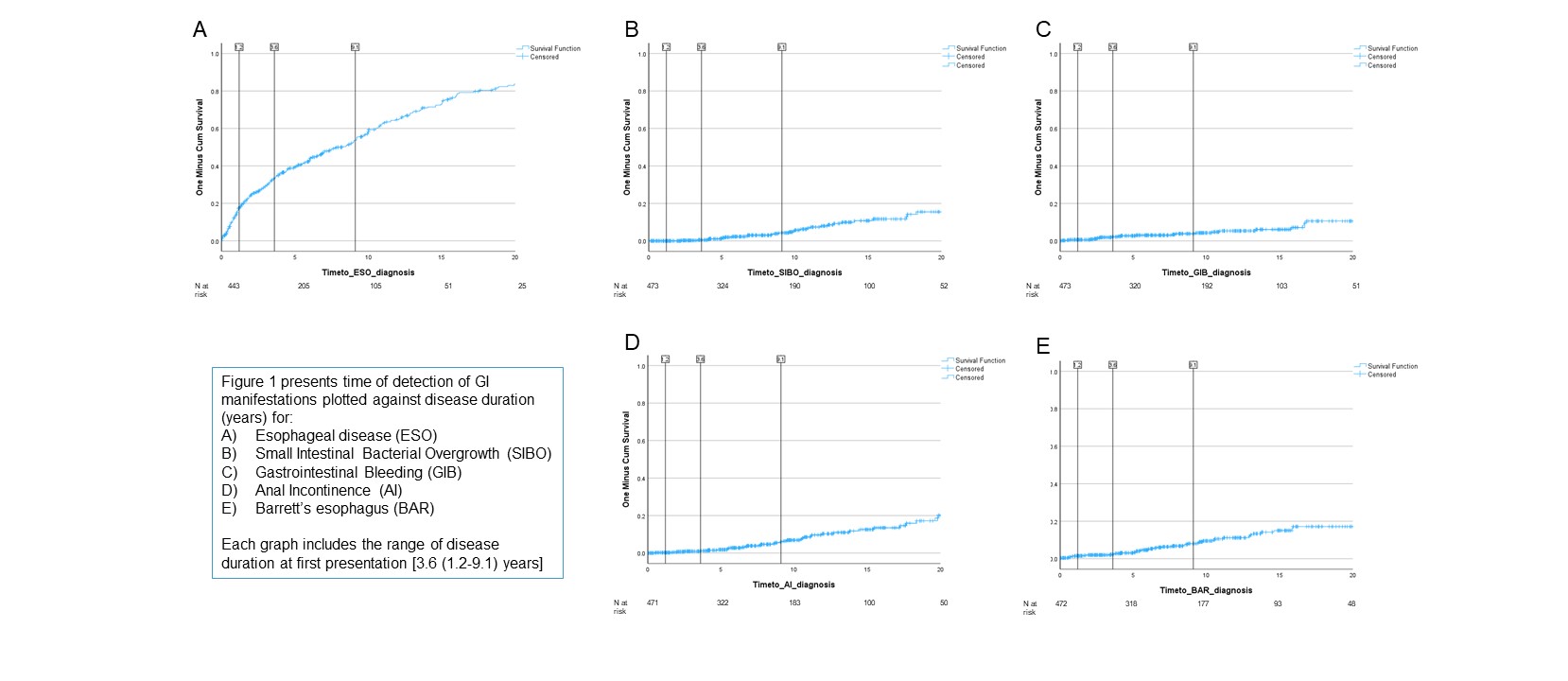 Table 1. Baseline characteristics of the included population stratified by severity of the GI involvement based on UCLA GIT 2.0 score.
Table 1. Baseline characteristics of the included population stratified by severity of the GI involvement based on UCLA GIT 2.0 score.
To cite this abstract in AMA style:
Bruni C, Klöti J, Tatu A, Stamm L, Dobrota R, Elhai M, Becker M, Muraru S, Sauer G, Hoffmann-Vold A, Distler O, Mihai C. Digesting the data: tracking gastro-intestinal manifestations in systemic sclerosis over time [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/digesting-the-data-tracking-gastro-intestinal-manifestations-in-systemic-sclerosis-over-time/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/digesting-the-data-tracking-gastro-intestinal-manifestations-in-systemic-sclerosis-over-time/

.jpg)