Session Information
Date: Tuesday, October 28, 2025
Title: (1780–1808) Osteoarthritis & Joint Biology – Basic Science Poster
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Osteoarthritis (OA) is characterized by a pro-inflammatory joint microenvironment, including elevated levels of endogenous pattern recognition receptor ligands in synovial fluid. Notably, both lipopolysaccharide (LPS) and its soluble co-receptor, soluble CD14 (sCD14), are increased in OA synovial fluid, and sCD14 levels correlate with disease severity. sCD14 facilitates LPS transfer to Toll-like receptor 4 (TLR4), potentiating immune responses to otherwise sub-threshold ligand levels. Although this mechanism has been demonstrated in myeloid cells and synovial fibroblasts, whether sCD14 can enhance LPS-driven inflammatory activation of human chondrocytes, at the low levels found in OA joints, remains unclear.
Methods: Primary human articular chondrocytes (hACs) were isolated from non-arthritic knee cartilage (obtained through the National Disease Research Interchange) and treated with LPS at OA-relevant (1 ng/mL) or supraphysiologic (100 ng/mL) concentrations, with or without sCD14 (2 µg/mL). NF-κB activation was assessed via Western blot, inflammatory gene expression by qPCR, and IL-8 and sCD14 secretion by ELISA (n=2–4). To assess CD14 dependency of the responses, C28/I2 chondrocytes (Merck) were treated with anti-CD14 antibody in parallel experiments.
Results: Disease-relevant concentrations of LPS (1 ng/mL) alone did not increase IL-8 secretion by hAC; however, co-treatment with sCD14 significantly elevated IL-8 protein levels compared to either condition alone (Fig. 1A). Expression of IL-8, CCL5, and CXCL10 was moderately increased with 1 ng/ml LPS, with trends toward enhancement by sCD14. In contrast, supraphysiologic LPS (100 ng/mL) induced robust inflammatory gene expression that was not further enhanced by sCD14, indicating a threshold effect (Fig. 1B-D). NF-κB activation was modestly affected by sCD14, suggesting involvement of additional signaling pathways (Fig. 1E, F). We further observed that chondrocytes did not secrete sCD14 under basal or LPS-stimulated conditions, supporting an exogenous origin of sCD14 (Fig. 2). Notably, sCD14 alone induced low-level inflammatory gene expression, suggesting it may function as a damage-associated molecular pattern in chondrocytes.In a therapeutic approach, CD14 blockade abrogated NF-κB activation induced by high-dose LPS, regardless of sCD14 presence, but had no effect on IL-8 expression. Importantly, at the OA-relevant concentration of 1 ng/mL LPS, CD14 inhibition reversed the sCD14-mediated increase in IL-8, effectively dampening inflammation (Fig. 3A, B).
Conclusion: sCD14 enhances chondrocyte sensitivity to disease-relevant concentrations of LPS, promoting inflammatory activation in a CD14-dependent manner. These findings identify sCD14 as a potential amplifier of chondrocyte-inflammatory responses in OA and support the potential of CD14 blockade to mitigate low-grade inflammation in cartilage.
 hAC were stimulated with 1 and 100 ng/ml LPS with and without 2 µg/ml sCD14. A) IL-8 secretion was determined by ELISA. Expression of B) IL-8, D) CCL5, and E) CXCL10 were determined by qPCR. Activation of NFkB-p65 was measured by western blot after E) 30 and F) 60 min. n= 2-4 donors, mean±SD. 2-way ANOVA and Tukey’s post hoc.
hAC were stimulated with 1 and 100 ng/ml LPS with and without 2 µg/ml sCD14. A) IL-8 secretion was determined by ELISA. Expression of B) IL-8, D) CCL5, and E) CXCL10 were determined by qPCR. Activation of NFkB-p65 was measured by western blot after E) 30 and F) 60 min. n= 2-4 donors, mean±SD. 2-way ANOVA and Tukey’s post hoc.
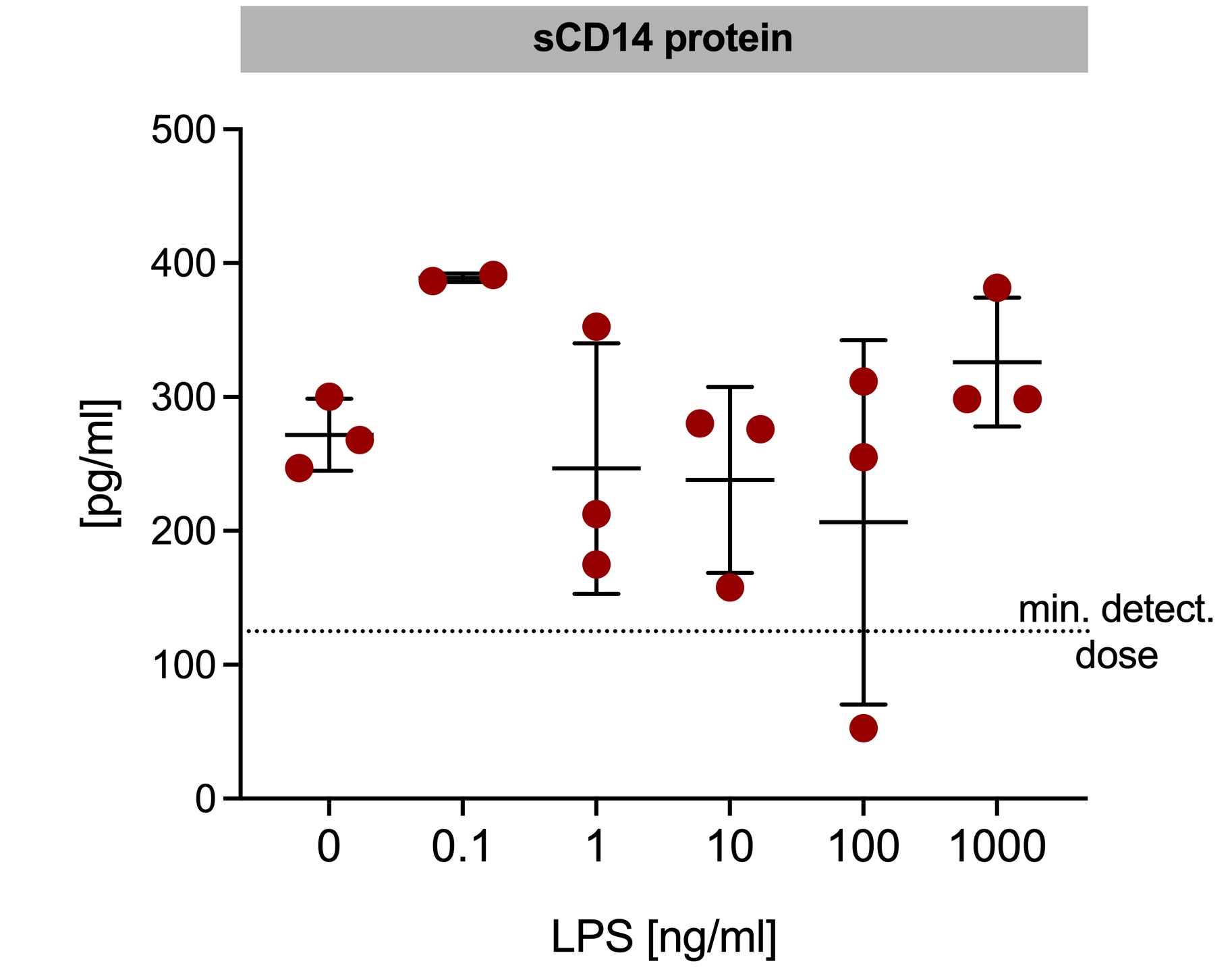
.jpg) sCD14 was determined in supernatants form hAC stimulated with increasing LPS concentrations. n = 2-3 donors, mean±SD.
sCD14 was determined in supernatants form hAC stimulated with increasing LPS concentrations. n = 2-3 donors, mean±SD.
.jpg) C28/I2 cells were stimulated with 1 and 100 ng/ml LPS±sCD14, with and without anti-CD14 (αCD14, clone 18D11). Anti-CD14 prevented A) NFκB activation by high-dose LPS independent of sCD14 (1h) and reduced the sCD14-dependent increase in B) IL-8 expression after low-dose LPS+sCD14 (24h). n= 2-3, mean±SD. 2-way ANOVA and Dunnett’s multiple comparisons test.
C28/I2 cells were stimulated with 1 and 100 ng/ml LPS±sCD14, with and without anti-CD14 (αCD14, clone 18D11). Anti-CD14 prevented A) NFκB activation by high-dose LPS independent of sCD14 (1h) and reduced the sCD14-dependent increase in B) IL-8 expression after low-dose LPS+sCD14 (24h). n= 2-3, mean±SD. 2-way ANOVA and Dunnett’s multiple comparisons test.
To cite this abstract in AMA style:
Rapp A, Sharp K, Hu B, Scanzello C. Soluble CD14 Amplifies Chondrocyte Inflammatory Responses to Lipopolysaccharide and Is Targetable by Anti-CD14 Therapy [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/soluble-cd14-amplifies-chondrocyte-inflammatory-responses-to-lipopolysaccharide-and-is-targetable-by-anti-cd14-therapy/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/soluble-cd14-amplifies-chondrocyte-inflammatory-responses-to-lipopolysaccharide-and-is-targetable-by-anti-cd14-therapy/
