Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Endothelial cell (EC) dysfunction is a key driver of cardiovascular (CV) complications in rheumatoid arthritis (RA), yet mechanisms underlying EC dysfunction in RA are not fully understood. Citrulline (CIT) and malondialdehyde-acetaldehyde (MAA) are post-translational modifications (PTMs) that are increasingly expressed and co-localized in RA tissues. These PTMs synergistically activate macrophages, leading to pro-inflammatory and pro-fibrotic responses in vitro. Our lab recently demonstrated increased expression and co-localization of CIT and MAA in heart tissues of mice with collagen-induced arthritis (CIA) that precedes fibrosis, suggesting a role of these PTMs in promoting local CV inflammation. However, their effects on EC function has not been examined. This study aims to examine whether CIT and MAA induce inflammatory responses in human coronary artery endothelial cells (HCAECs).
Methods: Human U-937 monocytes were differentiated into macrophages (M0 cells) and stimulated for 48 hrs with 25 ng/mL of fibrinogen that was unmodified (FIB) or modified with MAA (FIB-MAA), CIT (FIB-CIT), or MAA and CIT (FIB-MAA-CIT) (Fig. 1). To assess crosstalk, macrophage supernatants were collected, and HCAECs were indirectly stimulated with these supernatants using 25% of total well volume for 8 hrs, 1 day, or 7 days. Concurrently, HCAECs were directly stimulated with FIB, FIB-MAA, FIB-CIT, or FIB-MAA-CIT. After stimulation, HCAEC supernatants were analyzed by ELISA and mRNA measured by qRT-PCR. Statistical analyses were performed using GraphPad Prism and statistical significance was achieved when p< 0.05 and data comparision was conducted by a two-way ANOVA and Tukey’s post hoc test.
Results: Direct EC stimulation with FIB-MAA-CIT resulted in significantly higher levels of mRNA expressions of proinflammatory mediators, including MCP-1, IL-6, ICAM-1, VCAM1 (Fig. 2) and protein secretion (Fig. 3) vs. direct stimulation with FIB-MAA, FIB-CIT, or FIB alone. Interestingly, indirect stimulation of HCAECs with macrophage supernatants showed a similar pattern of inflammatory responses as direct stimulation, but the magnitude of the response was remarkably higher with indirect stimulation compared to direct antigen stimulation, suggesting an additional souble mediator secreted by macrophages contributing to EC activation. Additionally, we observed an earlier activation at mRNA and protein levels for adhesion molecules than peaks for cytokines, confirming cellular cross communications in response to MAA and CIT.
Conclusion: This research provides novel insights into the mechanistic crosstalk between inflammatory cells and coronary EC activation in RA. The data suggest that proteins co-modified with MAA and CIT (enriched in RA tissues) act synergistically to induce inflammatory responses in HCAECs. These inflammatory responses in HCAECs are amplified through macrophage activation and secretion of soluble mediators. In addition to identifying these mediators, further research is needed to examine whether inhibition of MAA, CIT, or downstream signaling pathways attenuates HCAEC activation and reduces CV complications in RA.
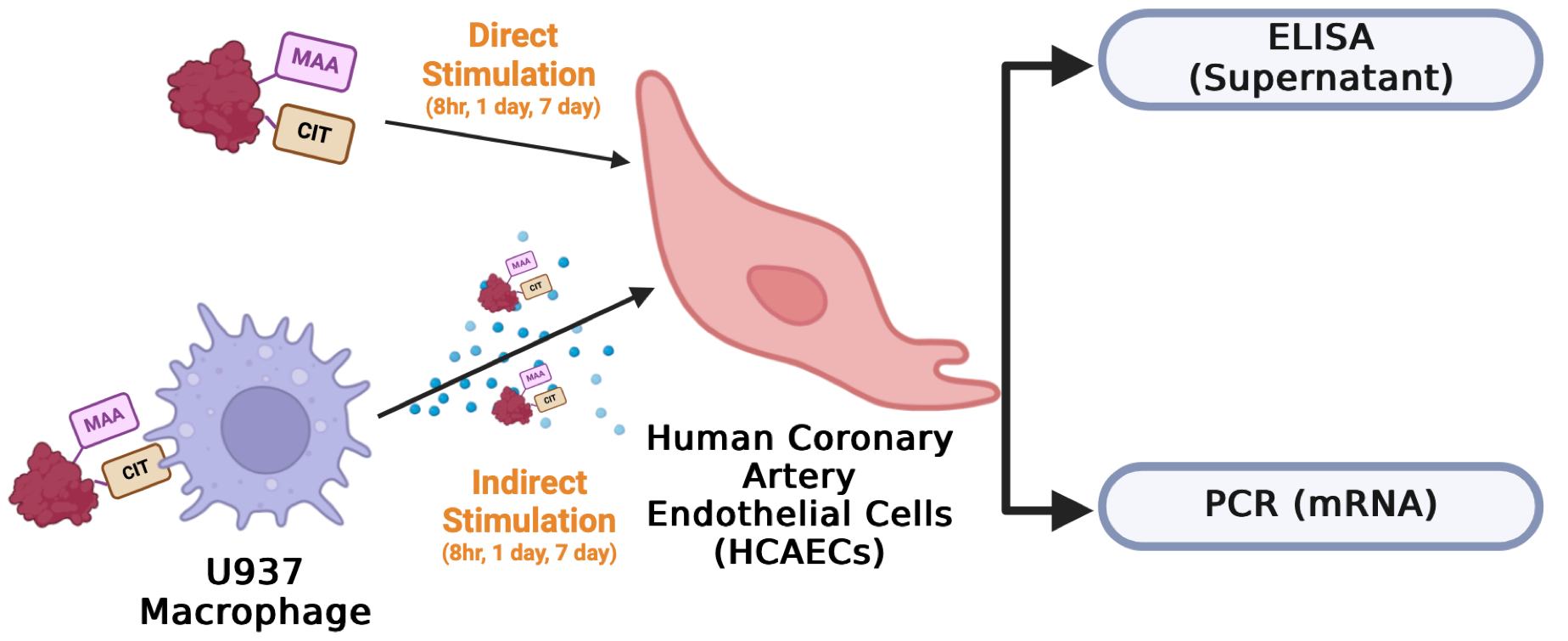 Fig 1. Study schema. HCAECs were either directly stimulated with CIT- and/or MAA-modified FIB or indirectly stimulated with supernatants from antigen-stimulated U-937 macrophages for 8 hours, 1 day, and 7 days. After stimulation, HCAEC supernatants were collected for ELISA, and mRNA was extracted for PCR.
Fig 1. Study schema. HCAECs were either directly stimulated with CIT- and/or MAA-modified FIB or indirectly stimulated with supernatants from antigen-stimulated U-937 macrophages for 8 hours, 1 day, and 7 days. After stimulation, HCAEC supernatants were collected for ELISA, and mRNA was extracted for PCR.
.jpg) Fig. 2. PCR Demonstrating Changes in HCAEC Gene Expression Following Direct Antigen Stimulation and Indirect Stimulation with Macrophage Supernatants. 8hr, 1 day, and 7 day mRNA expression was measured for A) MCP-1, B) IL-6, C) ICAM-1, and D) VCAM-1. Relative quotient (RQ) compared to media-cultured negative control cells were calculated and shown in y-axis. Two-way ANOVA was performed and the statistical differences between direct and indirect stimulation were shown in *: * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001. Statistical differences between CIT- and/or MAA-modified FIB compared with unmodified FIB were shown in ¶: ¶ p < 0.05; ¶¶ p < 0.01; ¶¶¶ p < 0.001; ¶¶¶¶ p < 0.0001.
Fig. 2. PCR Demonstrating Changes in HCAEC Gene Expression Following Direct Antigen Stimulation and Indirect Stimulation with Macrophage Supernatants. 8hr, 1 day, and 7 day mRNA expression was measured for A) MCP-1, B) IL-6, C) ICAM-1, and D) VCAM-1. Relative quotient (RQ) compared to media-cultured negative control cells were calculated and shown in y-axis. Two-way ANOVA was performed and the statistical differences between direct and indirect stimulation were shown in *: * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001. Statistical differences between CIT- and/or MAA-modified FIB compared with unmodified FIB were shown in ¶: ¶ p < 0.05; ¶¶ p < 0.01; ¶¶¶ p < 0.001; ¶¶¶¶ p < 0.0001.
.jpg) Fig. 3. ELISA Demonstrating Changes in HCAEC Protein Secretion Following Direct Antigen Stimulation and Indirect Stimulation with Macrophage Supernatants. 8hr, 1 day, and 7 day protein concentrations in supernatants were measured for A) MCP-1, B) IL-6, C) ICAM-1, and D) VCAM-1. Two-way ANOVA was performed and the statistical differences between direct and indirect stimulation were shown in *: * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001. Statistical differences between CIT- and/or MAA-modified FIB compared with unmodified FIB were shown in ¶: ¶ p < 0.05; ¶¶ p < 0.01; ¶¶¶ p < 0.001; ¶¶¶¶ p < 0.0001.
Fig. 3. ELISA Demonstrating Changes in HCAEC Protein Secretion Following Direct Antigen Stimulation and Indirect Stimulation with Macrophage Supernatants. 8hr, 1 day, and 7 day protein concentrations in supernatants were measured for A) MCP-1, B) IL-6, C) ICAM-1, and D) VCAM-1. Two-way ANOVA was performed and the statistical differences between direct and indirect stimulation were shown in *: * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001. Statistical differences between CIT- and/or MAA-modified FIB compared with unmodified FIB were shown in ¶: ¶ p < 0.05; ¶¶ p < 0.01; ¶¶¶ p < 0.001; ¶¶¶¶ p < 0.0001.
To cite this abstract in AMA style:
Zhou W, Johnson H, Duryee M, Sharp E, Hunter C, Johnson T, Alfaidi M, Anderson D, Bidasee K, Thiele G, Mikuls T. Citrullinated and Malondialdehyde-Acetaldehyde Modified Fibrinogen Activates Macrophages and Induces Inflammatory Responses in Coronary Endothelium [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/citrullinated-and-malondialdehyde-acetaldehyde-modified-fibrinogen-activates-macrophages-and-induces-inflammatory-responses-in-coronary-endothelium/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/citrullinated-and-malondialdehyde-acetaldehyde-modified-fibrinogen-activates-macrophages-and-induces-inflammatory-responses-in-coronary-endothelium/
