Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Rheumatoid arthritis (RA) patients are at increased risk for developing heart failure with preserved ejection fraction (HFpEF), which is characterized by impaired left ventricular relaxation. The mechanisms underlying RA-associated HFpEF remain unclear, but pro-fibrotic pathways are well-characterized. Citrulline (CIT) and malondialdehyde-acetaldehyde (MAA) are post-translational modifications (PTMs) that serve as autoantigens in RA. These mediators synergistically activate macrophages via NFkB and p38 signaling, leading to pro-inflammatory and pro-fibrotic responses in vitro. Our laboratory recently demonstrated that PTMs are over-expressed and co-localized in the cardiac tissues of RA-HF compared to non-RA HF patients. The objective of this study is to investigate whether cardiac fibrosis is also increased in RA-HF patients and to identify whether PTMs induce a pro-fibrotic responses in human coronary artery endothelial cells (HCAECs).
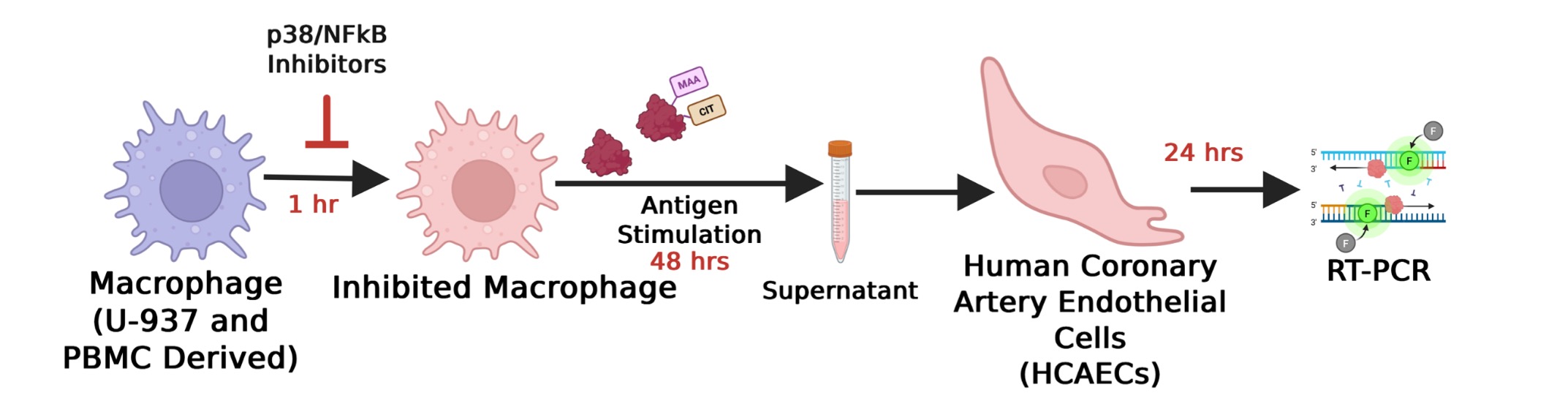
Methods: Left ventricular (LV) apex tissues from RA-HF patients (n=3) and age- and sex-matched non-RA HF controls (n=3) were stained with Masson’s trichrome. Cardiac fibrosis was measured by quantifying collagen deposition using ImageJ and analyzed using Student’s t test. In separate experiments, human U-937 monocytes (source) were differentiated into macrophages (Fig. 1). Macrophages were pre-incubated for 1 hr with either 1 μM BIRB-796 (p38 inhibitor), 50 μM BAY-11-7085 (NF-κB inhibitor), or media control. After that, macrophages were stimulated with 25 μg/mL of either unmodified fibrinogen (FIB) or PTM co-modified fibrinogen (FIB-MAA-CIT) for 48 hours. After stimulation, supernatants were collected and applied to HCAECs for 24 hours. HCAEC mRNA was isolated, and expression of fibrotic mediators (SMAD3, ACTA2, PDGF) was quantified by qRT-PCR. Fibrotic mediator expression was then compared using one-way ANOVA followed by Tukey’s post hoc test.
Results: Patients with RA-HF exhibited significantly increased perivascular fibrosis (p< 0.05) and a non-significant trend toward increased interstitial fibrosis (p=0.18) compared to non-RA HF controls (Fig. 2). Supernatants from FIB-MAA-CIT–stimulated macrophages significantly upregulated mRNA expressions of SMAD3, ACTA2, and PDGFB in HCAECs compared to FIB (p< 0.001). Pre-treatment with p38 or NF-κB inhibitors brought pro-fibrotic gene expression back to baseline levels (Fig. 3).
Conclusion: Our findings suggest that PTM–activated macrophages secrete soluble factors that induce marked pro-fibrotic responses in HCAECs. Those soluble factors are potentially contributing to increased collagen deposition, suggested by perivascular fibrosis observed in RA-HF cardiac tissues. Macrophage activation via p38 and NF-κB pathways appears critical in this process. Targeting PTMs or their downstream signaling pathways may represent a novel therapeutic strategy to attenuate HF burden in RA.
.jpg) Trichrome stain measuring collagen deposition in human heart tissues. Cardiac tissues from non-RA controls (non-RA HF controls) and RA patients (RA-HF) with Heart Failure (n=3/group). Collagen deposition (blue) were quantified using with Fiji plug-in and mean values from 3 images/patient calculated. Images were taken around blood vessels (perivascular fibrosis) and between cardiomyocytes (interstitial fibrosis). Representative image of 20X frame at left ventricle apex was shown. Data shown on graphs are mean ± standard deviation. Student’s t test was performed, and statistical differences were shown in *: * p < 0.05.
Trichrome stain measuring collagen deposition in human heart tissues. Cardiac tissues from non-RA controls (non-RA HF controls) and RA patients (RA-HF) with Heart Failure (n=3/group). Collagen deposition (blue) were quantified using with Fiji plug-in and mean values from 3 images/patient calculated. Images were taken around blood vessels (perivascular fibrosis) and between cardiomyocytes (interstitial fibrosis). Representative image of 20X frame at left ventricle apex was shown. Data shown on graphs are mean ± standard deviation. Student’s t test was performed, and statistical differences were shown in *: * p < 0.05.
.jpg) PCR measuring pro-fibrotic markers in HCAEC cells following stimulation with macrophage supernatants. Macrophage supernatants were collected post antigen stimulation (FIB or FIB-MAA-CIT) in the presence or absence of inhibitors (p38i or NFkBi). HCAEC gene expression was measured post 24-hour incubation for A) SMAD3, B) ACTA2, and C) PDGF. One-way ANOVA was performed and the statistical differences were shown in *: * p < 0.05; *** p < 0.001; **** p < 0.0001.
PCR measuring pro-fibrotic markers in HCAEC cells following stimulation with macrophage supernatants. Macrophage supernatants were collected post antigen stimulation (FIB or FIB-MAA-CIT) in the presence or absence of inhibitors (p38i or NFkBi). HCAEC gene expression was measured post 24-hour incubation for A) SMAD3, B) ACTA2, and C) PDGF. One-way ANOVA was performed and the statistical differences were shown in *: * p < 0.05; *** p < 0.001; **** p < 0.0001.
To cite this abstract in AMA style:
Aripova N, Zhou W, Johnson H, Duryee M, Sinanan K, Hunter C, Johnson T, Alfaidi M, Anderson D, Bidasee K, Thiele G, Mikuls T. Citrullinated and Malondialdehyde-Acetaldehyde Co-Modified Fibrinogen Activates Macrophages and Induces Pro-Fibrotic shift in Coronary Endothelium Phenotype [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/citrullinated-and-malondialdehyde-acetaldehyde-co-modified-fibrinogen-activates-macrophages-and-induces-pro-fibrotic-shift-in-coronary-endothelium-phenotype/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/citrullinated-and-malondialdehyde-acetaldehyde-co-modified-fibrinogen-activates-macrophages-and-induces-pro-fibrotic-shift-in-coronary-endothelium-phenotype/