Session Information
Date: Tuesday, October 28, 2025
Title: Abstracts: Immunological Complications of Medical Therapy (1728–1733)
Session Type: Abstract Session
Session Time: 11:15AM-11:30AM
Background/Purpose: While immune-related adverse events (irAEs) are a commonly reported complication of immune checkpoint inhibitor (ICI) therapy, factors associated with their development remain poorly defined. In this unique prospective cohort study, we explored lab data and patient-reported outcomes (PROs) associated with the probability of developing irAEs, focusing on those that could prompt a rheumatology consult. We aimed to identify high-risk patients who would potentially benefit from closer monitoring and early referral.
Methods: We enrolled cancer patients who were initiating ICI therapy. Demographics, PROs, and lab data were collected during routine clinical visits. irAEs were defined by health providers during routine practice. Rheumatologic-related irAEs included arthritis, myositis, myocarditis, rash, and dry mouth/eyes. Analysis was performed using chi-square or t-tests to compare the pre-treatment characteristics of patients who developed at least one irAE vs those who did not. Logistic regression models were used to evaluate if factors such as age, sex, treatment type (monotherapy with PD-1/PD-L1 inhibitors vs. combination therapy including CTLA-4 inhibitors), as well as baseline lab values (e.g., lymphocyte count, neutrophil-to-lymphocyte ratio, liver function tests), and PROs increased the odds of developing any irAE or rheumatologic irAEs. Cox regression models were used to assess whether time-varying assessments of these characteristics could identify those at more immediate risk.
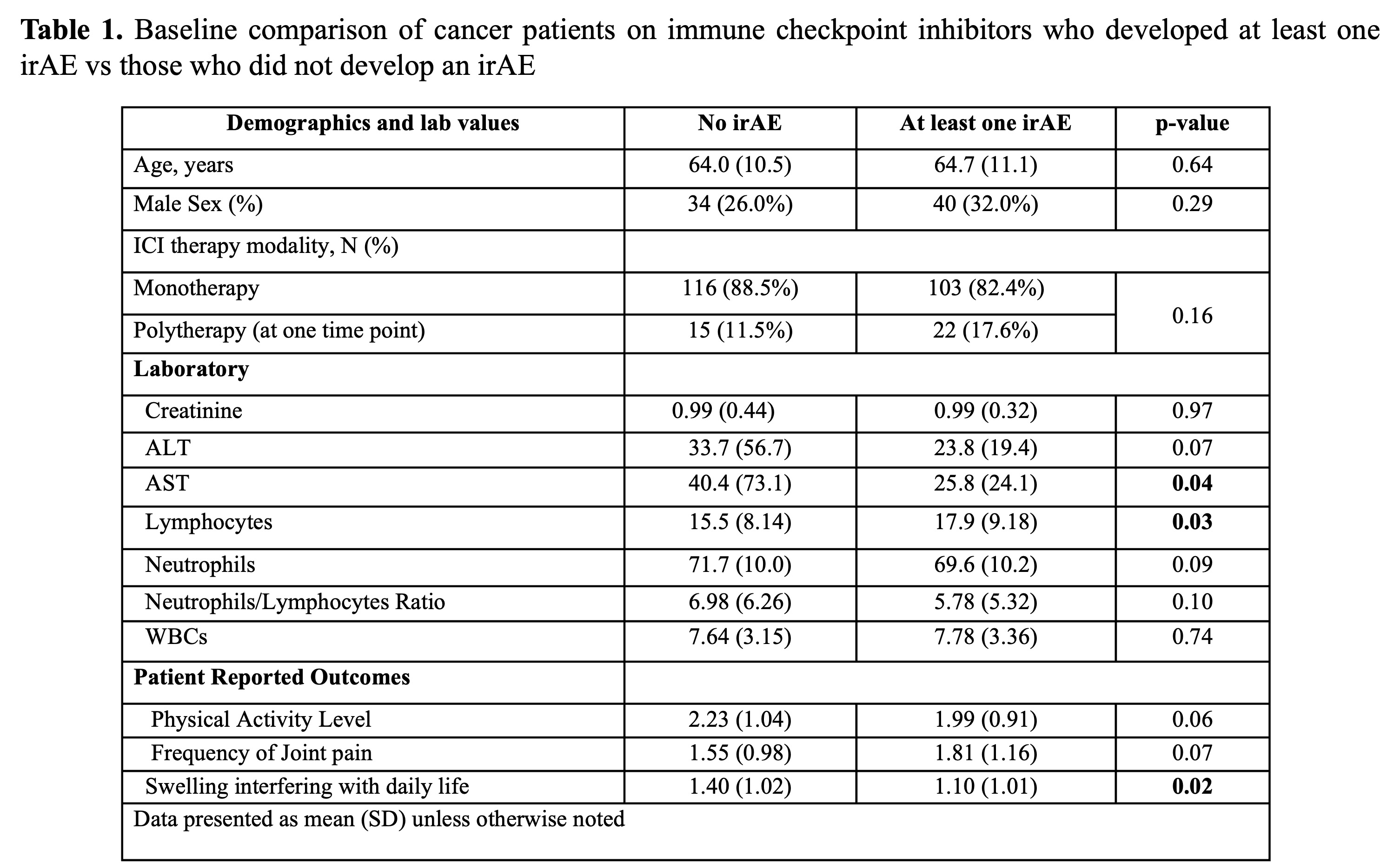
Results: Among 256 patients, 71.1% were male (n=182). Non-small lung cancer was the most common malignancy (23.1%), and the most common ICI was Pembrolizumab (40.9%). The incidence rate for any irAE was 11.3% (events/person-month) while it was 3.6% for rheumatologic irAEs. Notably, 50% of patients on ICIs had developed an irAE within 3.5 months, with 25% experiencing their first adverse event in the first month. At baseline, those who had irAEs had similar age, sex, rates of combination therapy with ICIs and PROs compared to those who did not. Patients who developed at least one irAE had higher lymphocyte counts (p=0.03) and a lower AST (p=0.04) (Table 1). Higher lymphocyte count (OR: 1.03; 95% CI: 1.0, 1.1; p=0.03) and lower AST (OR: 0.99; 95% CI: 0.98, 1.0; p=0.03) were associated with higher odds of developing an irAE, while use of combination immunotherapy (OR: 2.27; 95% CI: 1.11, 4.66; p=0.03) was associated with a higher odds of developing a rheumatologic irAE (Table 2). Most evaluated factors were unrelated to the immediate risk of developing irAEs.
Conclusion: In this large cohort study, PROs assessed at baseline and over time were poor predictors of irAE onset, including irAEs that would likely result in rheumatology referral. Consistent with prior literature, use of combination immune checkpoint inhibitor (ICI) therapy was associated with increased odds of irAEs, including rheumatologic irAEs. Higher baseline lymphocytes and lower AST levels were associated with an increased odds of irAE, and the neutrophil-to-lymphocyte ratio tended to be associated with irAEs, as has been observed in prior studies. Future studies should evaluate whether recent changes in lab values or PROs can predict the onset of irAEs.
To cite this abstract in AMA style:
Francis-Malave A, Laufer T, Baker J, Sacksith K, Batson M, Apostolidis S. Risk factors for the development of immune checkpoint inhibitor-related adverse events, including rheumatology-related presentations [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/risk-factors-for-the-development-of-immune-checkpoint-inhibitor-related-adverse-events-including-rheumatology-related-presentations/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-factors-for-the-development-of-immune-checkpoint-inhibitor-related-adverse-events-including-rheumatology-related-presentations/

.jpg)